OCR Specification focus:
‘Know the number of orbitals in s, p and d sub-shells, and the number of electrons that can fill these sub-shells.’
Introduction
Understanding sub-shells and electron counts is key to explaining how electrons are distributed within atoms. Each sub-shell has a defined number of orbitals and electron capacities.
Sub-shells in Electron Structure
An electron shell is divided into sub-shells, which determine how electrons occupy energy levels within an atom. Sub-shells define the number of orbitals available and their capacity to hold electrons.
Types of Sub-shells
Each shell, labelled by its principal quantum number (n), contains one or more sub-shells identified as s, p, d, or f.
They differ in:
The number of orbitals they contain
The maximum number of electrons they can hold
Their energy and shape
The s Sub-shell
The s sub-shell is the simplest and is found in every energy level. It contains one orbital, which can hold a maximum of two electrons.
Orbital: A region of space around an atomic nucleus where there is a high probability of finding an electron.
Each orbital contains two electrons with opposite spins, following the Pauli Exclusion Principle, which prevents two electrons from having identical quantum states.
The p Sub-shell
The p sub-shell appears from the second energy level (n = 2) onwards. It has three orbitals: px, py, and pz.
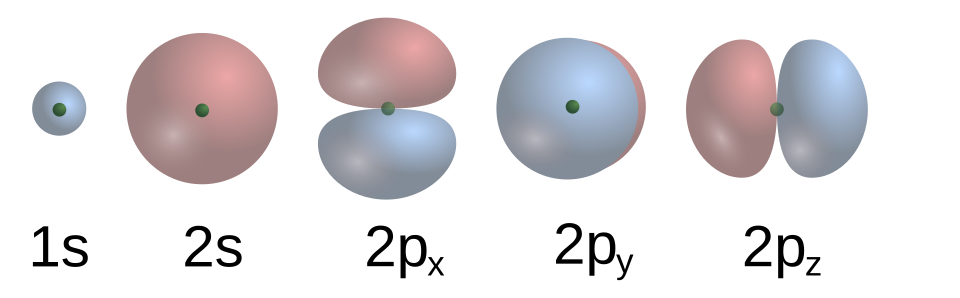
Labeled s and p orbital shapes highlighting that s has one orbital and p comprises three differently oriented orbitals (px, py, pz). Each orbital accommodates two electrons with opposite spins. This figure also shows an inner radial node for 2s, which is extra detail beyond the syllabus requirement. Source
Each p orbital:
Is dumbbell-shaped
Lies along a different axis (x, y, and z)
Holds two electrons
The p sub-shell therefore accommodates six electrons in total (3 orbitals × 2 electrons).
The d Sub-shell
The d sub-shell starts from the third energy level (n = 3). It has five orbitals, named dxy, dyz, dxz, dx²–y², and dz².
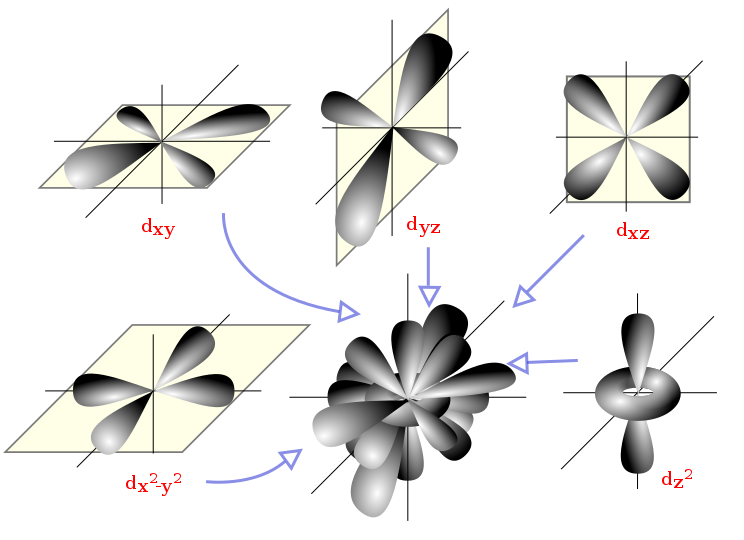
The d sub-shell comprises five orbitals with characteristic orientations: dxy, dyz, dxz, dx²–y², dz². Recognising these five distinct orbitals explains why the d sub-shell holds ten electrons in total (two per orbital). This figure focuses on shapes/orientations and does not depict energy ordering, which is beyond this subsubtopic. Source
Each orbital holds two electrons, so the d sub-shell can hold ten electrons in total.
This sub-shell plays a key role in transition metals, where partially filled d orbitals allow for variable oxidation states and complex bonding.
Electron Capacity by Sub-shell Type
Each sub-shell type holds a specific number of orbitals and therefore electrons:
s sub-shell: 1 orbital → 2 electrons
p sub-shell: 3 orbitals → 6 electrons
d sub-shell: 5 orbitals → 10 electrons
f sub-shell (beyond specification): 7 orbitals → 14 electrons
For OCR A-Level, focus is on s, p, and d sub-shells up to Z = 36 (Krypton).
Relationship Between Shells, Sub-shells, and Orbitals
Each principal energy level (shell) contains a specific combination of sub-shells:
n = 1: only an s sub-shell (1 orbital → 2 electrons)
n = 2: s and p sub-shells (4 orbitals → 8 electrons)
n = 3: s, p, and d sub-shells (9 orbitals → 18 electrons)
n = 4: s, p, d, and f sub-shells (16 orbitals → 32 electrons)
This systematic structure underpins the Periodic Table, as elements are ordered by their electron configurations, influencing their chemical behaviour.
Quantum Numbers and Sub-shell Properties
The distribution of electrons in sub-shells is described by quantum numbers, particularly:
The principal quantum number (n): identifies the shell
The azimuthal (angular momentum) quantum number (l): identifies the sub-shell type
Values of l correspond to sub-shells:
l = 0 → s sub-shell
l = 1 → p sub-shell
l = 2 → d sub-shell
l = 3 → f sub-shell
The number of orbitals in each sub-shell is given by:
Number of Orbitals in Sub-shell (l) = 2l + 1
l = Azimuthal quantum number (dimensionless)
Thus:
s sub-shell (l = 0): 2(0) + 1 = 1 orbital
p sub-shell (l = 1): 2(1) + 1 = 3 orbitals
d sub-shell (l = 2): 2(2) + 1 = 5 orbitals
Since each orbital holds two electrons, the total number of electrons per sub-shell = 2 × (2l + 1).
Pauli Exclusion Principle
This principle restricts the number of electrons in each orbital.
Pauli Exclusion Principle: No two electrons in an atom can have the same set of four quantum numbers. Therefore, each orbital holds a maximum of two electrons with opposite spins.
This ensures a structured filling of orbitals and prevents overlap of identical electron states.
Implications of Sub-shell Capacities
Knowing sub-shell electron counts helps in:
Constructing electron configurations for elements
Understanding periodic trends such as ionisation energy and atomic size
Predicting bonding behaviour and chemical reactivity
Sub-shell filling explains the division of the Periodic Table:
s-block: Groups 1–2, outer electrons in s sub-shell
p-block: Groups 13–18, outer electrons in p sub-shell
d-block: Transition metals, electrons in d sub-shell
This relationship shows how sub-shell structure determines an element’s position and properties.
Key Points
s sub-shell: 1 orbital, 2 electrons
p sub-shell: 3 orbitals, 6 electrons
d sub-shell: 5 orbitals, 10 electrons
Each orbital holds 2 electrons with opposite spins
Sub-shell capacities define atomic structure and chemical behaviour
Mastery of these relationships enables accurate determination of electron configurations and deeper understanding of atomic structure in A-Level Chemistry.
FAQ
Sub-shells differ because each corresponds to a different angular momentum quantum number (l).
The number of orbitals in a sub-shell is calculated using the formula 2l + 1.
As l increases (from s to p to d), the number of orbital orientations increases, reflecting the greater spatial complexity of electron movement. This explains why s has one orbital, p has three, and d has five.
The shape of an orbital affects how close an electron can approach the nucleus.
s orbitals are spherical, allowing electrons to penetrate close to the nucleus, giving them lower energy.
p and d orbitals have directional lobes, leading to slightly higher energies. Thus, shape influences both the electron density distribution and the energy hierarchy within shells.
Each orbital can accommodate two electrons due to the Pauli Exclusion Principle, which states that no two electrons in an atom can have identical quantum numbers.
The only way to differentiate two electrons in the same orbital is by their opposite spins, one spinning clockwise and the other anticlockwise.
Each principal quantum number (n) determines how many sub-shells are present in that shell.
The number of sub-shells equals the value of n.
For example:
n = 1 → 1 sub-shell (s)
n = 2 → 2 sub-shells (s, p)
n = 3 → 3 sub-shells (s, p, d) This systematic increase explains the growing complexity of higher shells.
The d sub-shell contributes to the chemical properties of transition metals.
Partially filled d orbitals allow for variable oxidation states.
They also participate in forming coordinate bonds and complex ions, where lone pairs from ligands overlap with empty or half-filled d orbitals. These properties explain the rich chemistry and colour diversity of transition metal compounds.
Practice Questions
State the number of orbitals in a p sub-shell and the maximum number of electrons that can occupy it.
(2 marks)
1 mark: States that there are three orbitals in a p sub-shell.
1 mark: States that the maximum number of electrons in a p sub-shell is six (two per orbital).
Describe the relationship between electron shells, sub-shells, and orbitals up to the fourth energy level. In your answer, include how many orbitals and electrons are possible in each energy level.
(5 marks)
1 mark: States that each energy level (shell) is made up of sub-shells (s, p, d, f).
1 mark: Correctly identifies that each orbital can hold two electrons with opposite spins.
1 mark: States that the first shell (n=1) has 1 sub-shell (s) → 1 orbital → 2 electrons.
1 mark: States that the second shell (n=2) has 2 sub-shells (s and p) → 4 orbitals → 8 electrons.
1 mark: States that the third shell (n=3) has 3 sub-shells (s, p, d) → 9 orbitals → 18 electrons, and the fourth shell (n=4) includes f → 16 orbitals → 32 electrons.
(Any correct, clearly structured response that includes all key figures for orbitals and electrons across the four shells gains full marks.)

