OCR Specification focus:
‘Deduce electron configurations of s- and p-block ions up to Z = 36 given atomic number and ionic charge.’
Understanding electron configurations of s- and p-block ions is essential for predicting chemical behaviour, charge formation, periodic trends, and the stability of the resulting electronic structures.
Electron Configurations of s- and p-Block Ions
Electron configurations describe how electrons are distributed across atomic orbitals, and this arrangement governs the formation of ions in the s- and p-block. Ions form when atoms lose or gain electrons to achieve more stable electronic structures, often resembling noble gas configurations. For OCR A-Level Chemistry, students must be able to deduce electron configurations for ions with atomic numbers up to 36, using sub-shell notation and understanding the effect of ionic charge on electron number.
The Basis of Ion Formation in the s- and p-Block
s- and p-block elements form ions through predictable patterns related to their group number.
Group 1 and 2 metals (s-block): lose electrons to form cations.
Groups 15–17 non-metals (p-block): gain electrons to form anions.
Group 18 elements: virtually never form ions due to full outer shells.
Electrons are always removed or added from/to the highest-energy occupied sub-shell, which corresponds to the outermost shell for s- and p-block elements.
Understanding Electron Loss in s-Block Cations
s-block metals form ions by losing electrons from their outermost s orbital. This process creates cations with reduced electron counts but unchanged proton numbers.
When the term cation is first introduced, it requires a definition.
Cation: A positively charged ion formed when an atom loses one or more electrons.
After an electron configuration is determined, the cation’s configuration must reflect the removal of electrons from the highest occupied shell, not the order of sub-shell filling.
Normal electrons in the s-block are removed as follows:
Loss of one electron → Group 1 elements form 1+ ions.
Loss of two electrons → Group 2 elements form 2+ ions.
Key points for s-block ions:
Electrons are removed from the ns sub-shell first.
No d or f orbitals are involved for elements up to Z = 36.
Electron Gain in p-Block Anions
Non-metals in the p-block typically form anions by gaining electrons to fill their p sub-shells. These elements move towards the stable configuration of the nearest noble gas.
When the term anion is first introduced, it requires a definition.
Anion: A negatively charged ion formed when an atom gains one or more electrons.
Students must be able to assign electrons to the 2p, 3p, and 4p sub-shells appropriately, depending on atomic number and the ionic charge.
General patterns include:
Group 17 elements gain one electron → 1− ions.
Group 16 elements gain two electrons → 2− ions.
Group 15 elements gain three electrons → 3− ions (less common but examinable).
Using Sub-Shell Notation for Ion Configurations
Electron configurations for ions must always be written in sub-shell notation, e.g. 2s² 2p⁶, not in box notation or dot notation.
Identify the atomic number to determine the number of protons.
Calculate the electron number in the ion:
For cations → subtract the ionic charge.
For anions → add the ionic charge.
Write the neutral atom configuration first using the standard filling order (1s → 2s → 2p → 3s → 3p → 4s → 3d → 4p).
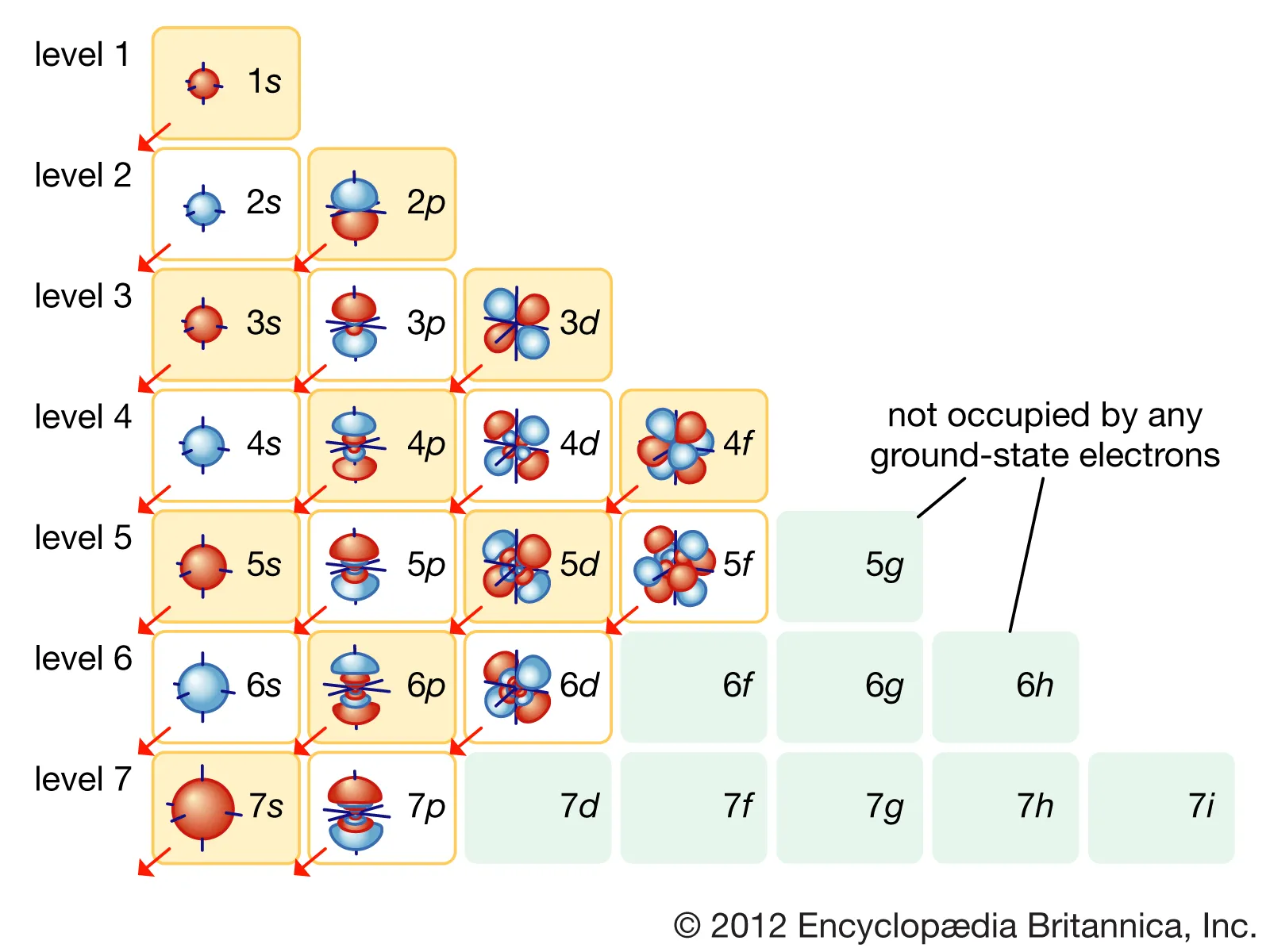
Caption: This diagram shows the order in which electrons fill atomic orbitals, illustrating energy progression and the placement of s, p, d, and f subshells. It helps visualise why electrons occupy orbitals in this sequence when determining electron configurations. Some subshells shown extend beyond the s- and p-block requirements for this topic. Source
Adjust the configuration by removing or adding electrons strictly from/to the highest principal quantum number (n).
This adjustment is critical; although 4s fills before 3d, electrons are removed from 4s before 3d only in transition metals — but this does not apply here because the sub-topic is restricted to s- and p-block ions.
Important Principles for Determining Ion Configurations
s- and p-block ions exhibit predictable behaviour. Students should internalise the following facts:
The outermost shell (highest n value) is always where electron addition or removal occurs.
No d electrons are involved for any element ≤ Z = 36 when forming simple s- or p-block ions.
A stable configuration often matches the nearest noble gas (He, Ne, Ar, Kr).
The effective number of electrons in the highest-energy p sub-shell governs the reactivity and ion formation tendencies.
Recognising Stability in Ion Configurations
The concept of stability is linked to achieving full s and p sub-shells. When an ion reaches configurations such as:
ns² np⁶ (full outer shell), or
1s² for hydrogen or lithium ions,
it attains a structure that reflects noble gas stability.
When the term noble gas configuration appears for the first time, a definition is necessary.
Noble gas configuration: An electron arrangement with a completely filled outer s and p sub-shell, giving exceptional stability.
Visualising Electron Redistribution
Although diagrams are not required in these notes, students should mentally picture how electrons shift between shells and sub-shells. Clear visualisation reinforces understanding of:
The sequential structure of shells: n = 1 to n = 4.
The boundaries of s and p sub-shell capacities:
s sub-shell → 1 orbital → 2 electrons.
p sub-shell → 3 orbitals → 6 electrons.
The directional flow of electrons (loss or gain) during ion formation.
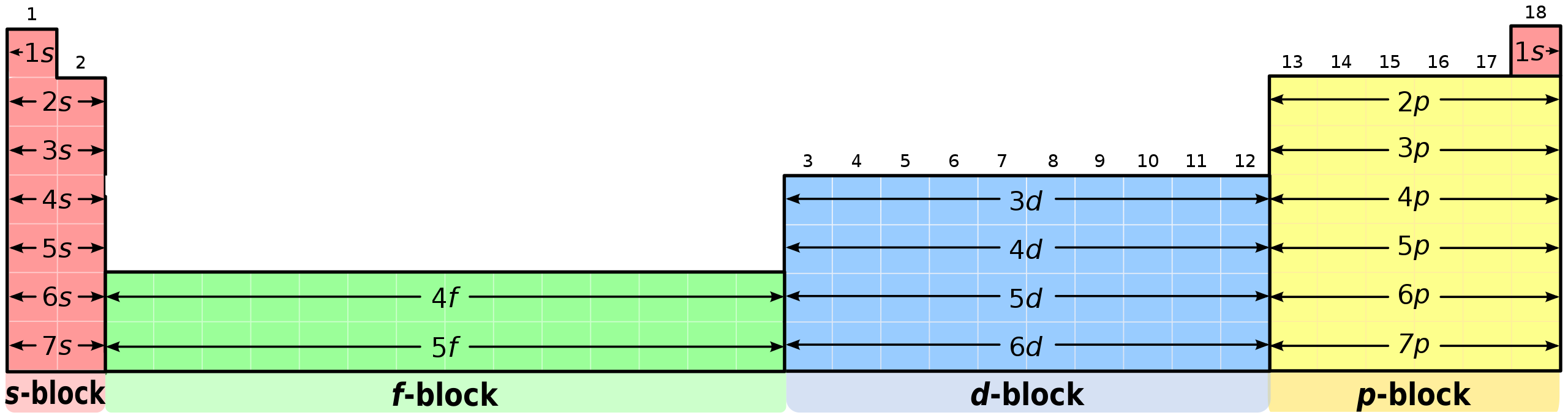
This diagram highlights the s- and p-block regions of the periodic table, supporting identification of which elements form the ions discussed in this topic. The d- and f-blocks appear but are not required for the OCR specification on s- and p-block ion configurations. Source
These patterns enable quick deduction of correct ion configurations when ionic charges and atomic numbers are provided.
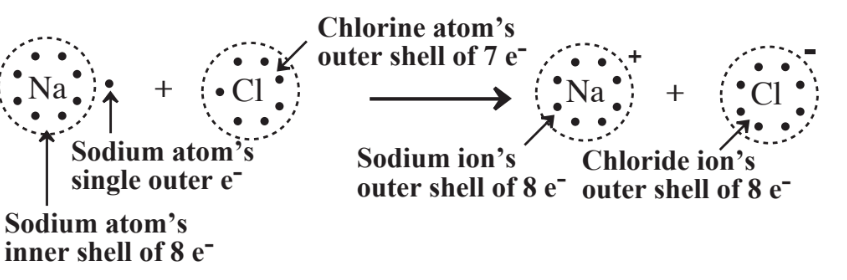
This diagram illustrates electron transfer between sodium and chlorine to form Na⁺ and Cl⁻, showing how electron gain and loss create stable noble-gas-like configurations. Although the figure also introduces ionic bonding, only the electron-transfer aspect is relevant to this subtopic. Source
FAQ
You always adjust the electron count before writing or modifying the configuration. Determine the ionic charge first, then remove or add electrons accordingly.
After this, place electrons into sub-shells following the filling order and the rule that electrons are lost from the outermost shell (highest n value) first. This ensures the configuration reflects the true structure of the ion, not the atom.
For elements up to Z = 36, the 3d sub-shell is only occupied in transition metals.
s- and p-block elements fill and adjust electrons exclusively within s and p sub-shells because:
Their outermost electrons are in s or p orbitals only.
Ion formation always involves the highest n value, which is never the 3d sub-shell for these elements.
Some p-block elements have sub-shell arrangements that allow both electron gain and electron loss in different conditions.
For example:
Group 15 and 16 elements may form less common positive ions by losing p electrons.
Metallic p-block elements (like Sn or Pb, though beyond Z = 36) demonstrate this most strongly.
Within Z ≤ 36, this principle explains why certain p-block species show variable oxidation states.
Effective nuclear charge affects how strongly electrons are held in outer orbitals.
A higher effective nuclear charge:
Makes it harder for atoms to lose electrons.
Increases the likelihood of electron gain for non-metals.
This helps explain trends such as:
s-block metals forming positive ions readily.
p-block non-metals gaining electrons to complete the p sub-shell.
A noble gas configuration corresponds to fully filled s and p sub-shells, creating a balanced and symmetrical distribution of electrons.
This stability arises because:
Completely filled sub-shells minimise electron repulsion.
No partially filled orbitals remain, lowering the atom’s energy.
Achieving this arrangement is the driving force behind the formation of many s- and p-block ions up to Z = 36.
Practice Questions
Magnesium forms an ion with a charge of 2+.
(a) State the electron configuration of the Mg²⁺ ion using sub-shell notation.
(b) Explain why magnesium forms a 2+ ion in terms of its electron structure.
(2 marks)
(a) Mg²⁺ electron configuration: 1s² 2s² 2p⁶
1 mark for correct configuration with all sub-shells shown.
(b) Explanation: magnesium loses two electrons from the 3s sub-shell to achieve a stable noble gas arrangement.
1 mark for stating electrons are lost from 3s.
Award full marks only if the configuration in (a) is correct.
Chlorine forms a chloride ion, Cl⁻, while aluminium forms an aluminium ion, Al³⁺.
(a) Deduce the full electron configuration for each ion using sub-shell notation.
(b) Explain, with reference to sub-shells and stability, why these ions form their respective charges.
(c) Identify the nearest noble gas configuration each ion achieves.
(5 marks)
(a) Electron configurations:
Cl⁻: 1s² 2s² 2p⁶ 3s² 3p⁶ (1 mark)
Al³⁺: 1s² 2s² 2p⁶ (1 mark)
(b) Explanation:
Cl gains one electron to fill the 3p sub-shell, reaching full outer-shell stability. (1 mark)
Al loses three electrons (from 3s and 3p) to return to a full 2p subshell, achieving stability. (1 mark)
(c) Noble gas configurations:
Cl⁻ achieves argon configuration. (0.5 mark)
Al³⁺ achieves neon configuration. (0.5 mark)

