OCR Specification focus:
‘Define covalent and dative bonds; construct dot-and-cross diagrams; interpret average bond enthalpy as a measure of covalent bond strength.’
Covalent bonding is central to understanding molecular structure and chemical reactivity. These notes introduce covalent and dative bonding, show how electron-pair sharing forms molecules, and explain how average bond enthalpy provides a comparative measure of bond strength across different covalent species.
Covalent Bonding
Covalent bonding arises when atoms share pairs of electrons to achieve greater stability. This type of bonding dominates in molecules composed of non-metals and is strongly related to electronegativity and orbital overlap.
Covalent bond is introduced here.
Covalent bond: A bond formed by the sharing of a pair of electrons between two non-metal atoms.
Covalent bonds result in the formation of discrete molecules, each with a specific arrangement of nuclei and shared electrons. Electron sharing can be equal or unequal, depending on electronegativity differences, although the OCR specification emphasises the structure and nature of the electron-pair interaction rather than polarity.
Dot-and-cross diagrams
Dot-and-cross diagrams are pictorial representations that show how atoms share electrons to form covalent bonds. They use different symbols to distinguish the electrons originating from each atom. Although simplified, they help visualise:
Shared electron pairs (bonding pairs)
Lone (non-bonding) electron pairs
The number of bonds formed by each atom
The octet rule where applicable
These diagrams reinforce that each covalent bond consists of two electrons, contributed by one or both bonding atoms.

Dot-and-cross diagram of hydrogen chloride (HCl), showing a shared pair of electrons between hydrogen and chlorine and three lone pairs on chlorine. This visual reinforces how a single covalent bond and lone pairs are represented in electron-pair diagrams. The diagram uses dots rather than different symbols for each atom, but the underlying idea of shared and non-bonding pairs is identical to OCR’s required dot-and-cross approach. Source
Dative (Coordinate) Covalent Bonding
In some molecules and ions, one atom donates both electrons to the shared pair. This still forms a covalent bond but differs in its origin.
Dative covalent bond is introduced here.
Dative covalent bond (coordinate bond): A covalent bond in which both electrons in the shared pair originate from the same atom.
A common example is the formation of ammonium ions from ammonia and hydrogen ions. Dative bonds are treated identically to normal covalent bonds once formed, but their formation highlights the flexibility of electron pair donation.
Singly donated pairs often use arrows in diagrams (from donor to acceptor), although the resulting bond behaves as an ordinary covalent bond in all structural and energetic contexts.
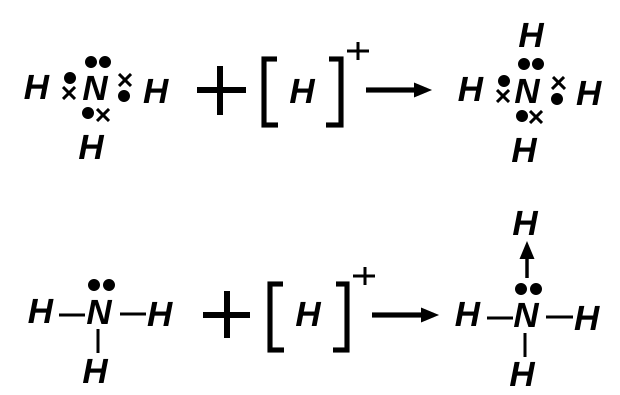
Diagram illustrating a coordinate (dative) covalent bond, with an arrow showing a lone pair from the donor atom forming a bond to the acceptor atom. This highlights that both electrons in the bond come from the same atom, even though the resulting bond is indistinguishable from other covalent bonds. The image may also mention Lewis acids and bases, which is useful context but not required knowledge for OCR A-Level Chemistry. Source
Bond Strength and Average Bond Enthalpy
To compare covalent bonds quantitatively, chemists use average bond enthalpies. These values reflect the energy required to break one mole of a specific bond type in the gaseous state, averaged across multiple compounds because bond strengths vary with molecular environment.
Before this can be explored, the term is introduced.
Average bond enthalpy: The mean energy required to break one mole of a given covalent bond in gaseous molecules, producing gaseous atoms.
Average bond enthalpies provide insight into molecular stability and reactivity. Higher values indicate stronger bonds requiring more energy to break. This concept supports predictions of reaction energetics and helps explain trends in molecular structures and chemical behaviour.
A sentence separating definitions and equations is placed here, as required.
Bond Enthalpy (D) = Energy required to break one mole of bonds
D = Bond enthalpy, measured in kJ mol⁻¹
Because actual bond enthalpies differ depending on molecular surroundings—for example, C–H bonds in methane differ subtly from those in ethane—averaged values enable consistent comparison across compounds. OCR requires students to understand the meaning of these values and their use as a measure of covalent bond strength, rather than to calculate enthalpy changes in full thermochemical cycles.
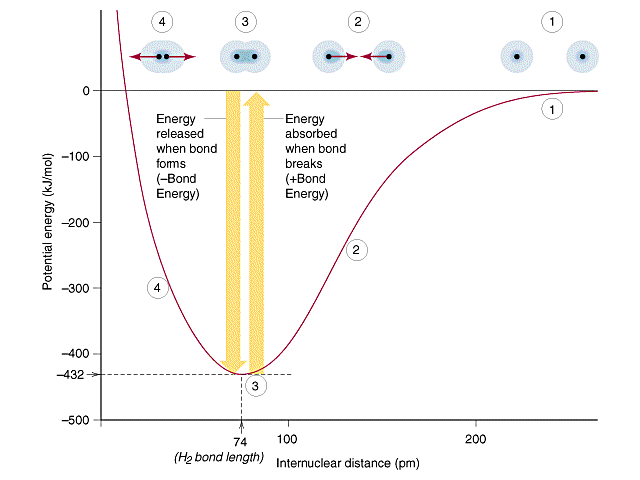
Potential-energy curve for the H–H bond showing how energy changes with internuclear distance. The minimum of the curve marks the equilibrium bond length, while the depth of the well corresponds to the bond energy required to separate the atoms. The diagram may also label regions of net attraction and repulsion, which extend slightly beyond the OCR specification but help explain why stronger bonds are shorter and have higher bond enthalpies. Source
Factors Influencing Covalent Bond Strength
Bond strength is influenced by several conceptual factors that relate back to electronic structure and orbital interaction. These factors help explain why some covalent bonds have higher bond enthalpies than others.
Bond length
Shorter bonds tend to be stronger. As atomic radii decrease, the shared electrons are held more closely to both nuclei, enhancing electrostatic attraction within the bond. This is why bonds involving smaller atoms (e.g., H–H, F–F) often possess high bond enthalpies.
Bond order
Bond order refers to the number of shared electron pairs between atoms:
Single bonds involve one shared pair
Double bonds share two pairs
Triple bonds share three pairs
Increasing bond order generally leads to:
Greater electron density between nuclei
Shorter bond length
Higher bond enthalpy
Thus, a C≡C triple bond is significantly stronger than a C=C double bond.
Electronegativity
Differences in electronegativity can influence bond strength by affecting how tightly electrons are held within the shared region. Polar covalent bonds often exhibit enhanced ionic character, which can strengthen or weaken the bond depending on the size and charge distribution of the atoms involved.
Multiple Covalent Bonds and Their Energetics
In molecules featuring multiple bonds, bond enthalpies cannot simply be multiplied to find total energies because each bond in a multiple bond differs slightly in strength. However, OCR focuses on understanding:
Single, double, and triple covalent bonds
How increasing shared electron pairs affects bond strength
Why bond enthalpies used in thermochemical calculations are averages
Students should be able to interpret values from data tables and relate them to structural features of molecules.
Covalent Structures and Strong Bonds
Covalent bonding underpins both simple molecular substances and giant covalent structures. In giant lattice structures, such as those found in carbon allotropes (diamond, graphite), the network of strong covalent bonds extends throughout the structure, giving rise to:
High melting and boiling points
Insolubility in most solvents
Hardness or, in some cases, electrical conductivity
While this subsubtopic does not require detailed study of lattice structures, recognising that strong covalent bonds give rise to these bulk physical properties reinforces the conceptual link between bond strength and material behaviour.
Summary of Key Points Aligned to OCR Requirements
A covalent bond involves shared pairs of electrons.
A dative covalent bond involves both electrons donated by one atom.
Dot-and-cross diagrams illustrate how electron pairs are shared.
Average bond enthalpy measures the energy needed to break a covalent bond and reflects bond strength.
Factors such as bond length, bond order, and electronegativity influence bond strength.
FAQ
Stronger covalent bonds form when atomic orbitals overlap more effectively, increasing electron density between the nuclei.
Greater overlap occurs when:
Orbitals are similar in energy
Orbitals are oriented correctly for maximum interaction
Atomic distances allow strong attraction without too much repulsion
Poor overlap weakens the bond because shared electrons are held less effectively between the atoms.
Dative bond formation depends on the availability of a lone pair and the presence of an atom with an empty orbital capable of accepting it.
Favourable conditions include:
A donor atom with a concentrated lone pair (e.g., N or O)
A highly electron-deficient acceptor (e.g., H+, metal cations)
Minimal steric hindrance that allows orbital alignment
Molecules lacking either a suitable donor or acceptor cannot form coordinate bonds.
Average bond enthalpies combine values from multiple compounds, smoothing out variations caused by molecular structure.
Individual molecules may show:
Different electron distribution
Varying degrees of bond polarity
Structural strain or stabilisation effects
These factors make actual bond energies slightly different from averaged table values.
Bond strength can change if molecular geometry alters the position or direction of orbital overlap.
Examples include:
Bent or distorted structures reducing effective overlap
Linear or symmetrical arrangements enhancing electron sharing
Lone pairs compressing bond angles, increasing repulsion and slightly weakening bonds
Even small changes in geometry can subtly shift bond enthalpy.
Double and triple bonds contain different combinations of sigma and pi bonding, each with distinct strengths.
A double bond includes one sigma bond and one pi bond, while a triple bond has one sigma and two pi bonds.
Pi bonds arise from sideways orbital overlap, which is less efficient than sigma overlap.
As a result, bond energies increase with bond order but not in simple multiples of single-bond values.
Practice Questions
Explain the difference between a covalent bond and a dative (coordinate) covalent bond.
(2 marks)
Covalent bond: formed when each atom supplies one electron to a shared pair. (1 mark)
Dative (coordinate) covalent bond: both electrons in the shared pair come from the same atom. (1 mark)
Covalent bond strength can be interpreted using average bond enthalpies.
(a) Explain what is meant by average bond enthalpy. (2 marks)
(b) Discuss two factors that influence the strength of a covalent bond, using appropriate chemical reasoning. (3 marks)
(5 marks)
(a)
Average bond enthalpy is the mean energy required to break one mole of a specified covalent bond in gaseous molecules. (1 mark)
It is an average because bond energies vary depending on the molecular environment. (1 mark)
(b)
Any two of the following, each with correct reasoning:
Bond length: shorter bonds are stronger as nuclei are closer, increasing attraction between nuclei and shared electrons. (1 mark per well-explained factor)
Bond order: higher bond order (e.g., double or triple bonds) increases electron density between nuclei, strengthening the bond.
Electronegativity difference: differences in electronegativity can strengthen or weaken a bond depending on how electrons are distributed.
Maximum 3 marks for part (b).

