OCR Specification focus:
‘Define ionic bonding as electrostatic attraction between oppositely charged ions; explain giant ionic lattices and resulting physical properties.’
Ionic bonding involves strong attractions between ions formed by electron transfer. Understanding lattice structure and resulting physical properties is essential for explaining the behaviour of ionic compounds.
Ionic Bonding and Lattice Structure
Formation of Ions
Ionic bonding begins with the formation of cations and anions, arising from electron transfer between atoms with significantly different electronegativities.
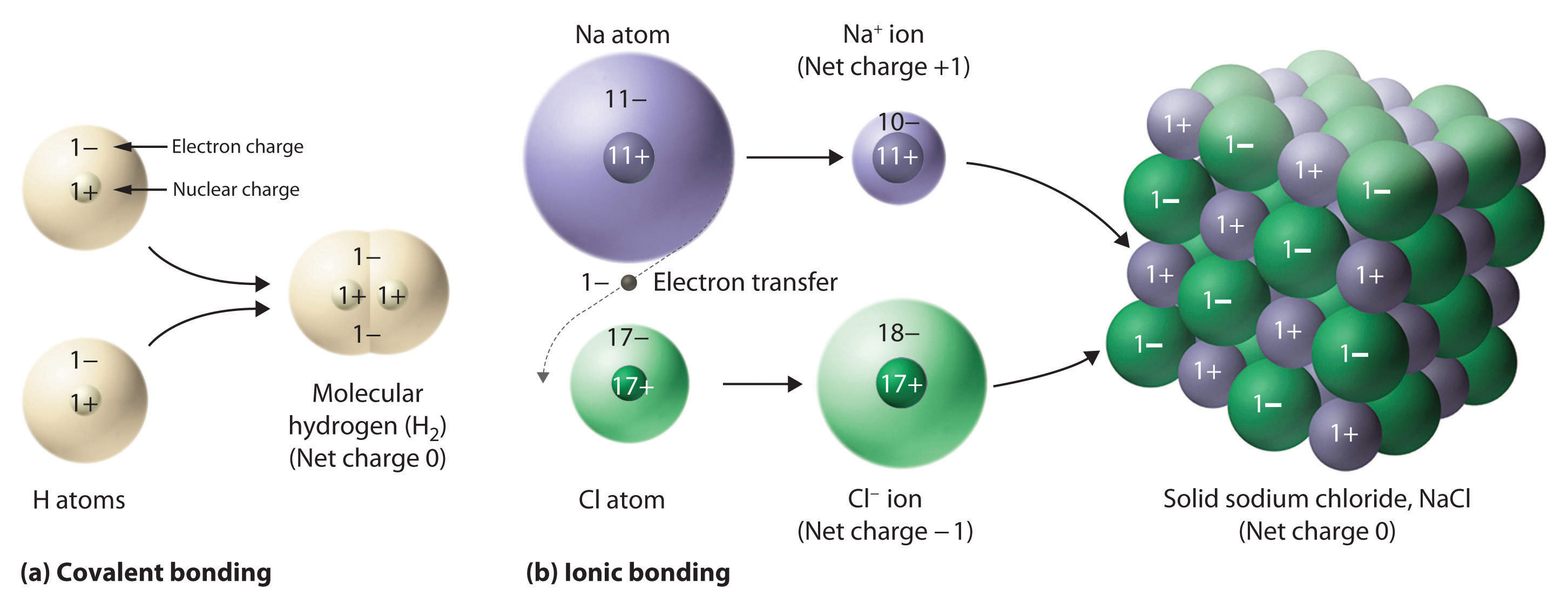
This diagram shows electron transfer from sodium to chlorine forming Na⁺ and Cl⁻ ions, which assemble into an extended ionic lattice. The left-hand covalent example contains extra content not required by OCR. Source
Metals typically lose electrons to form positive ions, while non-metals gain electrons to form negative ions. This transfer enables both species to achieve more stable electron configurations resembling noble gases.
Ionic Bonding: Electrostatic attraction between oppositely charged ions formed through electron transfer.
Once formed, cations and anions attract in all directions due to their opposite charges, creating an extended arrangement rather than discrete molecular units. This results in a giant three-dimensional ionic lattice.
A sentence comes here before the next definition block to maintain required spacing.
Giant Ionic Lattice: A continuous, regularly repeating structure of alternating cations and anions held together by strong electrostatic forces.
Energetics of Ionic Bonding
The strength of ionic bonding depends on ionic charge and ionic radius.
Higher charges produce stronger attractions, while smaller ions allow the ions to pack more closely, intensifying electrostatic forces. These factors help determine melting point, solubility, and other physical properties.
Structure of Ionic Lattices
Arrangement of Ions
In a giant ionic lattice, ions adopt positions that maximise attraction and minimise repulsion.
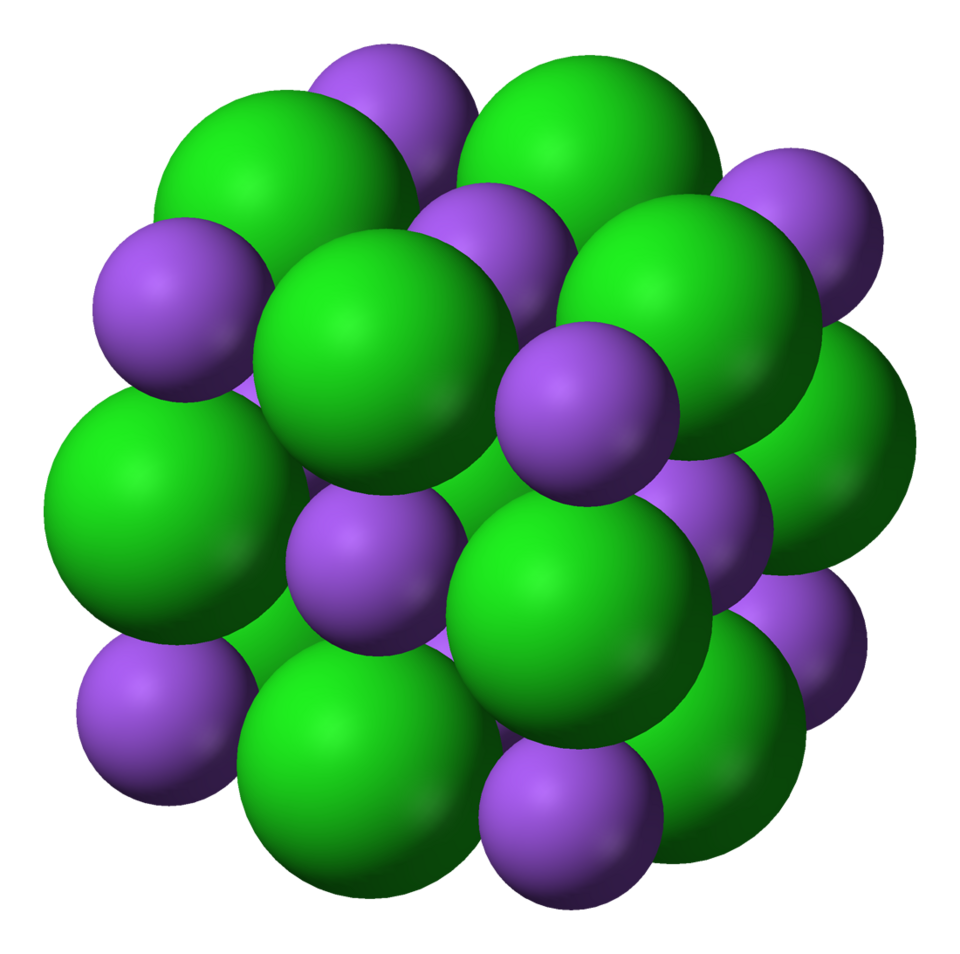
This 3D model shows the cubic arrangement of alternating ions in a sodium chloride lattice. It highlights the repeating three-dimensional structure responsible for the compound’s high stability and melting point. Source
Although the exact shape varies (e.g., sodium chloride structure, caesium chloride structure), all lattice types share key features:
A regular, repeating arrangement of ions.
Alternating positive and negative ions.
Strong ionic bonds extending throughout the lattice.
No individual molecules present—ionic compounds exist as continuous networks.
Characteristics of Lattice Structures
The structure of an ionic lattice is closely linked to the physical behaviour of ionic compounds.
Key characteristics include:
High stability due to strong electrostatic forces.
Three-dimensional bonding keeping ions fixed in place.
Directional uniformity, as the electrostatic attraction acts equally in all directions.
Physical Properties of Ionic Compounds
High Melting and Boiling Points
Ionic compounds exhibit high melting and boiling points because breaking the strong electrostatic attractions in the lattice requires substantial energy. The stronger the ionic bond—often influenced by higher ionic charges or smaller ionic radii—the higher the temperature needed to overcome these forces.
Electrical Conductivity
Electrical conductivity in ionic compounds varies between states:
Solid state:
Ions are fixed in position within the lattice.
No conductivity, as there are no mobile charged particles.
Molten state or aqueous solution:
The lattice breaks down, releasing free-moving ions.
Good conductors, due to mobile ions carrying electrical charge.
Molten ionic compounds and aqueous solutions of ionic compounds conduct electricity because the ions are mobile and can move to carry charge.
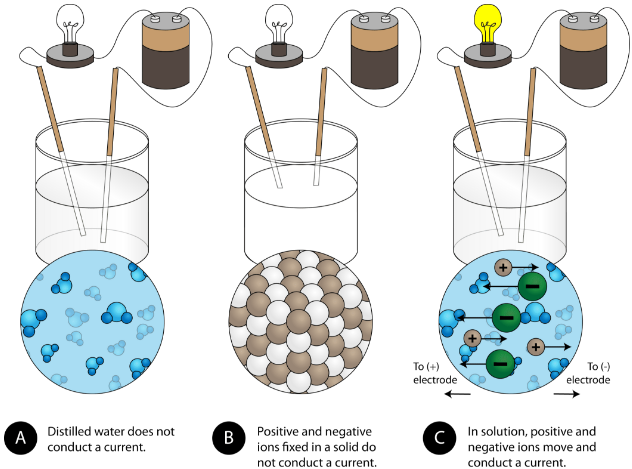
This diagram contrasts conductivity in distilled water, solid ionic compounds, and ionic solutions. It shows that only solutions with mobile ions complete the circuit and light the bulb. The distilled-water comparison offers extra context beyond OCR requirements. Source
Solubility in Water
Many ionic compounds dissolve in water because the partial charges on water molecules attract the ions in the lattice.
Water molecules:
Surround cations and anions.
Pull ions away from the lattice.
Separate and stabilise them in solution.
This process depends on the balance between lattice enthalpy (energy needed to break the ionic lattice) and hydration enthalpy (energy released when ions form attractions with water).
Brittleness
Ionic compounds are characteristically brittle rather than malleable.
When a force is applied:
Layers of ions may shift.
Ions of the same charge can be forced adjacent to one another.
Repulsion causes the lattice to fracture.
This brittleness results from the rigid, directional electrostatic forces and the inability of ions to slide without destabilising the structure.
Linking Structure to Properties
How Lattice Strength Determines Behaviour
The strength of the ionic lattice influences several observable features:
Thermal properties: Stronger lattices require more energy to melt or boil.
Solubility trends: Compounds with exceptionally high lattice enthalpies may be insoluble or only sparingly soluble.
Hardness: Ionic lattices resist deformation due to rigid ion arrangements.
Comparison with Other Bond Types
Although not assessed directly in this subsubtopic, it is useful to recognise that ionic bonding differs from covalent and metallic bonding in terms of particle arrangement, mobility of species, and characteristic behaviours such as electrical conductivity and melting points. Understanding these contrasts reinforces the distinctive features of ionic bonding.
Processes Involved in Ionic Bond Formation and Lattice Construction
Electron transfer creates ions with full outer shells.
Electrostatic attraction occurs instantly between oppositely charged ions.
Lattice assembly continues spontaneously as ions attract additional neighbouring ions.
Three-dimensional packing forms the giant ionic lattice, maximising attractive forces.
These processes collectively explain the formation, structure, and properties of ionic compounds according to the OCR specification.
FAQ
The distance between the nuclei of neighbouring ions determines how strongly they attract each other. Smaller ions pack more closely, increasing electrostatic attraction and strengthening the lattice.
As a result, compounds with smaller ions usually show higher melting points, greater hardness, and lower solubility if the lattice becomes very stable.
Solubility depends on the balance between lattice enthalpy and hydration enthalpy.
High hydration enthalpy (strong attraction between ions and water) favours dissolving.
High lattice enthalpy (very strong ionic bonding) can prevent the lattice breaking apart.
Compounds with highly charged or very small ions often remain insoluble due to their exceptionally strong lattices.
Ion charge density measures charge relative to ionic size. Ions with high charge density strongly attract their neighbours, creating tightly bound lattices.
This leads to:
Higher melting and boiling points
Lower volatility
Reduced solubility in many cases
Charge-dense ions also distort neighbouring electron clouds more effectively, influencing the compound’s overall stability.
The lattice adopted depends mainly on the relative sizes of the cation and anion.
When ions are of similar size, a cubic structure like caesium chloride may be favoured.
If the cation is significantly smaller, the sodium chloride structure becomes more stable.
The chosen arrangement maximises attraction and minimises repulsion between ions.
Ionic bonding is non-directional, meaning the electrostatic attraction acts equally in all directions.
However, the lattice arrangement is directional: ions occupy fixed positions.
When layers shift under stress, ions of the same charge may align, causing strong repulsion.
This sudden repulsion fractures the lattice, producing the characteristic brittleness of ionic solids.
Practice Questions
State what is meant by ionic bonding and explain why ionic compounds have high melting points.
(2 marks)
Ionic bonding is the electrostatic attraction between oppositely charged ions (1)
Large amounts of energy are required to overcome strong electrostatic forces in the giant ionic lattice, giving high melting points (1)
Sodium oxide, Na2O, is an ionic compound.
Explain, in terms of structure and bonding, why:
(a) solid sodium oxide does not conduct electricity,
(b) molten sodium oxide does conduct electricity, and
(c) sodium oxide is brittle when struck.
(5 marks)
(a) Solid Na2O does not conduct electricity:
Ions are held in fixed positions within the giant ionic lattice (1)
No mobile charged particles to carry charge (1)
(b) Molten Na2O conducts electricity:
Lattice breaks down when molten (1)
Ions become free to move and carry charge (1)
(c) Na2O is brittle:
Shifting layers forces like charges adjacent, causing repulsion and the lattice to fracture (1)

