OCR Specification focus:
‘Predict shapes and bond angles for up to six electron pairs using electron pair repulsion, including effects of lone pairs and common angles.’
Molecular shapes and bond angles are determined by the arrangement of electron pairs around a central atom, where repulsions between bonding and lone pairs control the three-dimensional geometry.
Molecular Geometry and the VSEPR Principle
The Valence Shell Electron Pair Repulsion (VSEPR) theory underpins the prediction of molecular shapes for A-Level Chemistry. It is based on the idea that electron pairs in the valence shell arrange themselves to minimise repulsion, producing specific molecular geometries and bond angles. This subsubtopic focuses on predicting shapes for molecules containing up to six electron pairs, recognising the influence of both bonding pairs and lone pairs.
VSEPR theory: A model stating that electron pairs around a central atom repel each other and adopt positions that minimise repulsion, determining molecular shape.
VSEPR provides a systematic approach for identifying geometry by examining the number and type of electron pairs present. Lone pairs exert stronger repulsion than bonding pairs, causing characteristic deviations from idealised bond angles in many molecules.
You should be able to predict shapes and bond angles for molecules with up to six electron pairs around a central atom.
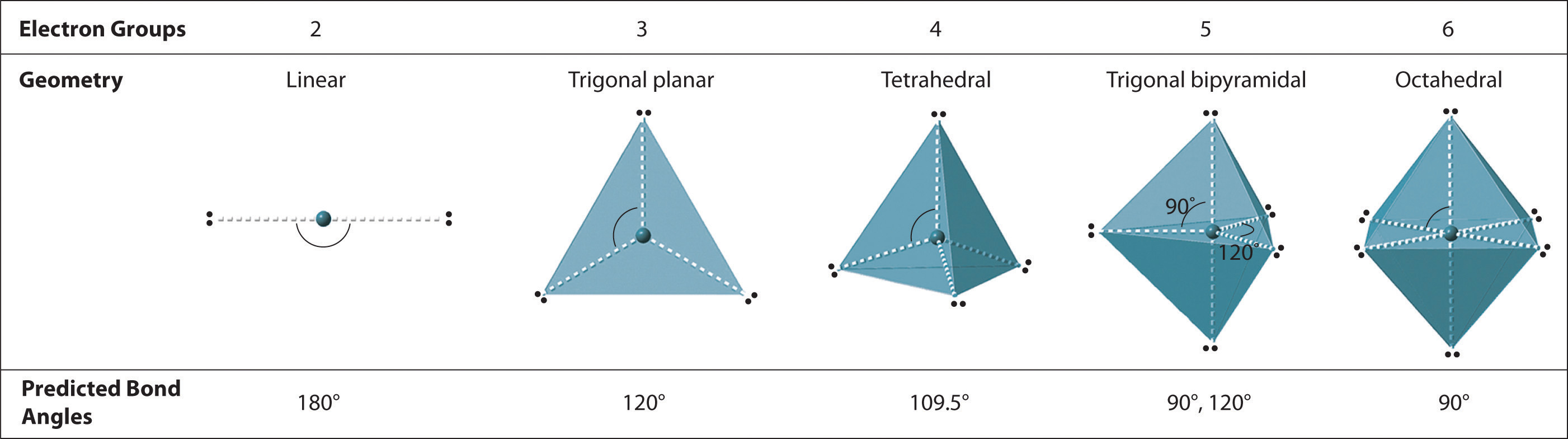
Diagram of the ideal electron‑pair geometries for 2–6 electron groups, illustrating linear, trigonal planar, tetrahedral, trigonal bipyramidal and octahedral shapes with their characteristic bond angles. Source
Electron Pair Repulsion and Its Consequences
Bonding Pairs vs Lone Pairs
Electron pair repulsion is not uniform. Different electron pair types influence molecular structure in specific ways:
Lone pairs > bonding pairs in repulsive strength.
Multiple bonds count as a single region of electron density despite containing more electrons.
Increasing numbers of lone pairs generally reduce bond angles between bonding pairs.
Because lone pairs occupy more space, they compress bond angles and distort ideal shapes. This effect becomes especially important when comparing real molecular angles with theoretical predictions.
Predicting Shapes Using Electron Pair Numbers
Determining Regions of Electron Density
To apply VSEPR:
Count the total number of regions of electron density (bonding pairs + lone pairs) around the central atom.
Choose the geometry corresponding to this number.
Adjust the name of the shape if lone pairs are present (e.g., trigonal pyramidal, bent, square pyramidal).
Between explanation and definition, the text should help students connect electron pair counts directly with real molecular geometry.
Electron pair: A region of electron density around an atom, consisting of either a bonding pair or a lone pair.
Ideal Geometries for 2–6 Electron Pairs
Two electron pairs
Arrangement: Linear
Typical bond angle: 180°
Lone pairs: Uncommon in stable two-pair molecules.
Three electron pairs
Arrangement: Trigonal planar
Ideal angle: 120°
With one lone pair: Bent shape, angle slightly <120°.
Four electron pairs
Arrangement: Tetrahedral
Ideal angle: 109.5°
One lone pair: Trigonal pyramidal, angle ~107°.
Two lone pairs: Bent, angle ~104.5°.
These variations demonstrate how lone pairs progressively compress bond angles.
Five electron pairs
Arrangement: Trigonal bipyramidal
Bond angles: 90°, 120°, 180°
One lone pair: Seesaw shape.
Two lone pairs: T-shaped.
Three lone pairs: Linear.
Six electron pairs
Arrangement: Octahedral
Bond angles: 90°, 180°
One lone pair: Square pyramidal.
Two lone pairs: Square planar.
These models allow students to predict reliable shapes and angles using only electron pair counts, aligning directly with the OCR specification.
Lone Pair Effects on Bond Angles
Why Lone Pairs Distort Shapes
Lone pairs occupy electron density closer to the nucleus and are more compressed, increasing repulsion with bonding pairs. As a result:
Bond angles decrease as lone pairs are added.
The distortion follows a general pattern:
Lone pair–lone pair > lone pair–bond pair > bond pair–bond pair.
This hierarchy explains the well-known progression of decreasing angles across tetrahedral-based shapes.
Common Angle Deviations
Several bond angles frequently appear in OCR exams:
109.5° → 107° → 104.5° as lone pairs increase around a tetrahedral arrangement.
120° → slightly less in trigonal planar systems with one lone pair.
90° angles in octahedral systems remain generally robust but may subtly reduce with lone pair presence.
These values should be recognised and memorised, as they are fundamental for accurate molecular geometry predictions.
Shapes for Molecules with Six or Fewer Electron Pairs
Summary of Key Shapes (Bullet-Point Format)
Linear (2 pairs)
– 180° bond angle
– Symmetrical electron distributionTrigonal planar (3 pairs)
– 120°
– Distorted to bent with one lone pairTetrahedral (4 pairs)
– 109.5°
– Trigonal pyramidal (1 lone pair)
– Bent (2 lone pairs)Trigonal bipyramidal (5 pairs)
– 90°, 120°, 180°
– Seesaw, T-shaped, linear (with increasing lone pairs)Octahedral (6 pairs)
– 90°, 180°
– Square pyramidal or square planar with lone pairs
These forms represent the full range of shapes required by the specification.
Applying VSEPR to Real Molecules
Understanding electron pair repulsion allows students to make precise qualitative predictions:
Identify central atom and total electron pairs.
Determine ideal geometry.
Adjust expected bond angles if lone pairs are present.
Name the resulting shape using standard terminology.
Between explanation and definition, the text should help students connect electron pair counts directly with real molecular geometry.
In the tetrahedral family, compare methane (CH₄), ammonia (NH₃) and water (H₂O).
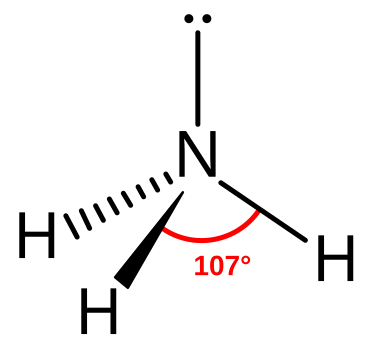
Molecular structure of ammonia showing three N–H bonds in a trigonal pyramidal shape and a lone pair on nitrogen, illustrating lone‑pair repulsion reducing bond angles. Source
In a trigonal bipyramidal arrangement, three positions are equatorial (120° apart in a plane) and two are axial (above and below, 90° to the plane).
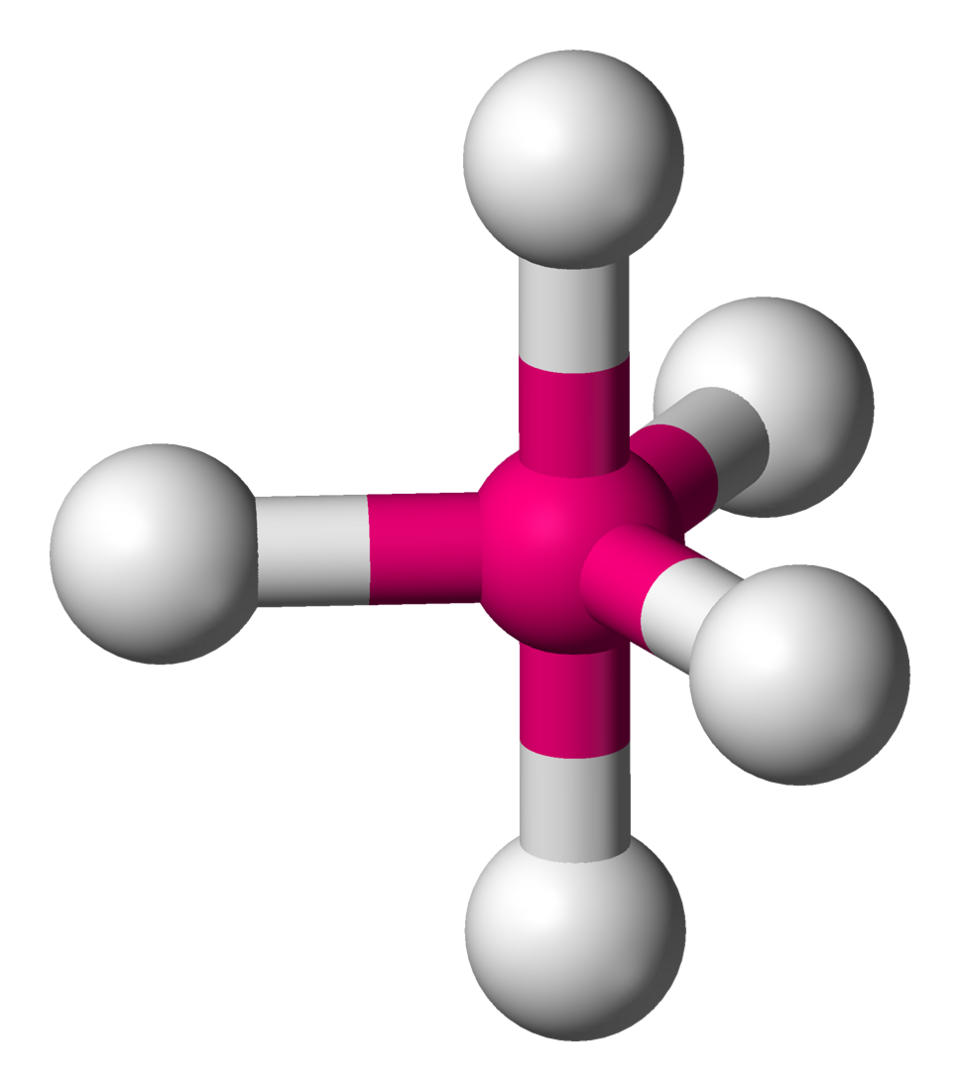
Ball‑and‑stick model of a trigonal bipyramidal molecule, showing three equatorial positions at 120° and two axial positions at 90°, supporting visualisation of bond‑angle differences. Source
FAQ
VSEPR theory treats double and triple bonds as a single region of electron density. This means they occupy one electron-pair domain, just like a single bond, even though they contain more electrons.
However, multiple bonds exert slightly stronger repulsion than single bonds. This can cause small adjustments in bond angles, especially when both single and multiple bonds are present around the same central atom.
These distortions do not change the basic predicted shape but may subtly affect exact angle values.
In a trigonal bipyramidal arrangement, equatorial positions experience less repulsion because they are separated by 120°, whereas axial positions sit between three 90° interactions.
Placing a lone pair equatorially minimises total repulsion because:
Lone pairs repel more strongly than bonding pairs.
Equatorial positions reduce the number of 90° repulsions.
This is why molecules like SF4 adopt a seesaw shape rather than placing the lone pair axially.
Electron-pair geometry considers all electron domains, but molecular shape considers only the positions of atoms.
For example:
Tetrahedral electron geometry can produce tetrahedral (CH4), trigonal pyramidal (NH3), or bent (H2O) shapes depending on lone pairs.
Lone pairs are invisible in molecular shape, so the same electron arrangement can give multiple distinct structures.
This distinction is crucial for explaining varied shapes from a single parent geometry.
More electronegative atoms pull bonding electrons closer, slightly altering repulsion patterns around the central atom.
This can:
Reduce repulsion between bonding pairs.
Allow lone pairs to dominate more strongly.
Shift angles by a few degrees compared with standard VSEPR predictions.
These effects do not change the predicted shape but can fine-tune the actual measured angles.
Lone pairs sit closer to the nucleus and occupy more concentrated regions of electron density, making them chemically accessible.
Their presence can:
Create regions of high electron density that attract electrophiles.
Increase polarity by distorting bond angles.
Influence intermolecular interactions, such as hydrogen bonding or dipole–dipole attraction.
Although VSEPR focuses on geometry, these reactivity differences arise directly from lone-pair positioning.
Practice Questions
Predict the shape and bond angle of a molecule with three bonding pairs and one lone pair around its central atom.
Explain your reasoning.
(2 marks)
Identifies shape as trigonal pyramidal. (1 mark)
States bond angle is approximately 107° and explains reduction from 109.5° due to lone-pair repulsion. (1 mark)
Sulphur tetrafluoride (SF4) has five electron pairs around the central sulphur atom: four bonding pairs and one lone pair.
Using VSEPR theory, explain fully:
(a) The electron-pair geometry around the sulphur atom.
(b) The molecular shape of SF4.
(c) Why the bond angles deviate from the ideal angles of the parent geometry.
(5 marks)
(a) Identifies electron-pair geometry as trigonal bipyramidal. (1 mark)
(b) Identifies molecular shape as seesaw. (1 mark)
(c) Explanation of bond-angle deviation:
Lone pair occupies an equatorial position to minimise repulsion. (1 mark)
Lone pair–bond pair repulsion is greater than bond pair–bond pair repulsion. (1 mark)
As a result, axial and equatorial bond angles are compressed from the ideal 90° and 120° values. (1 mark)

