OCR Specification focus:
‘Across Periods 2 and 3, electron configurations follow periodic patterns. Elements are classified into s-, p-, and d-blocks.’
Introduction
Electron configuration patterns across Periods 2 and 3 reveal predictable arrangements of electrons in sub-shells, enabling classification of elements into s-, p-, and d-blocks based on their highest-energy electrons.
Electron Configuration Across Periods 2 and 3
The arrangement of electrons in atoms is fundamental to understanding periodicity. As proton number increases across a period, electrons fill sub-shells in a specific sequence determined by increasing energy levels. This produces repeating patterns that form the basis for chemical periodicity.
When discussing electron configuration, the term electron configuration refers to the ordered distribution of electrons among energy levels and sub-shells according to quantum rules. The periodic table reflects this structure directly, meaning its layout is a visual map of the order in which electrons occupy orbitals.
Electron Configuration: The arrangement of electrons in an atom’s energy levels, sub-shells, and orbitals.
Electron configurations across Periods 2 and 3 follow a consistent pattern:
Period 2 elements fill the 2s and then the 2p sub-shells.
Period 3 elements fill the 3s and then the 3p sub-shells.
Sub-shells fill in order of increasing energy, which broadly follows the sequence 1s → 2s → 2p → 3s → 3p → 4s → 3d.
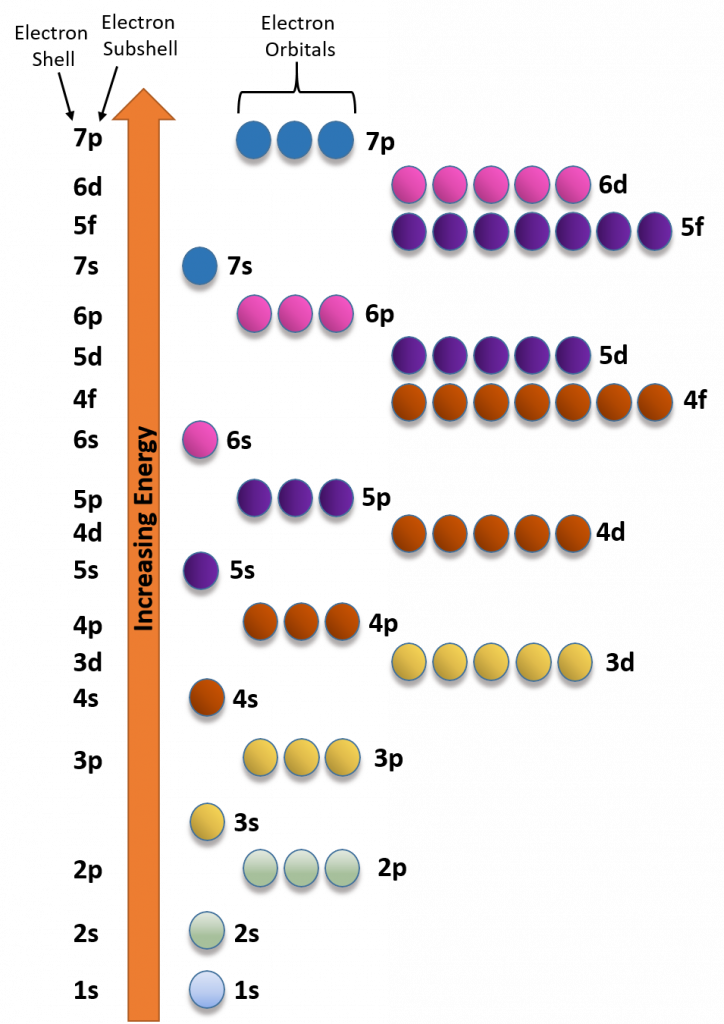
Diagram showing the relative energies of s, p, d and f sub-shells, reinforcing the electron filling order. Includes shells beyond Periods 2 and 3 but follows the same pattern. Source
The Aufbau Principle, Pauli Exclusion Rule and Hund’s Rule
Although these principles are not named explicitly in the OCR specification, they underpin the arrangement of electrons and therefore support understanding of sub-shell filling patterns:
Electrons occupy the lowest-energy available orbital first.
Each orbital holds a maximum of two electrons with opposite spins.
Within a set of degenerate (equal-energy) orbitals, electrons fill singly before pairing.
A normal sentence is included here to maintain correct spacing before any further structural explanation.
Sub-shell Structure and Filling Patterns
Across Periods 2 and 3, sub-shells follow predictable filling orders because:
Increasing nuclear charge across a period draws electrons closer, influencing energetic ordering.
The relative energies of s and p sub-shells remain consistent across these periods, leading to repeated sub-shell filling patterns.
Key Features of Sub-shell Filling
s-sub-shells contain a single orbital and hold up to two electrons.
p-sub-shells contain three orbitals and hold up to six electrons.
Each successive element increases proton number by one and introduces one additional electron.
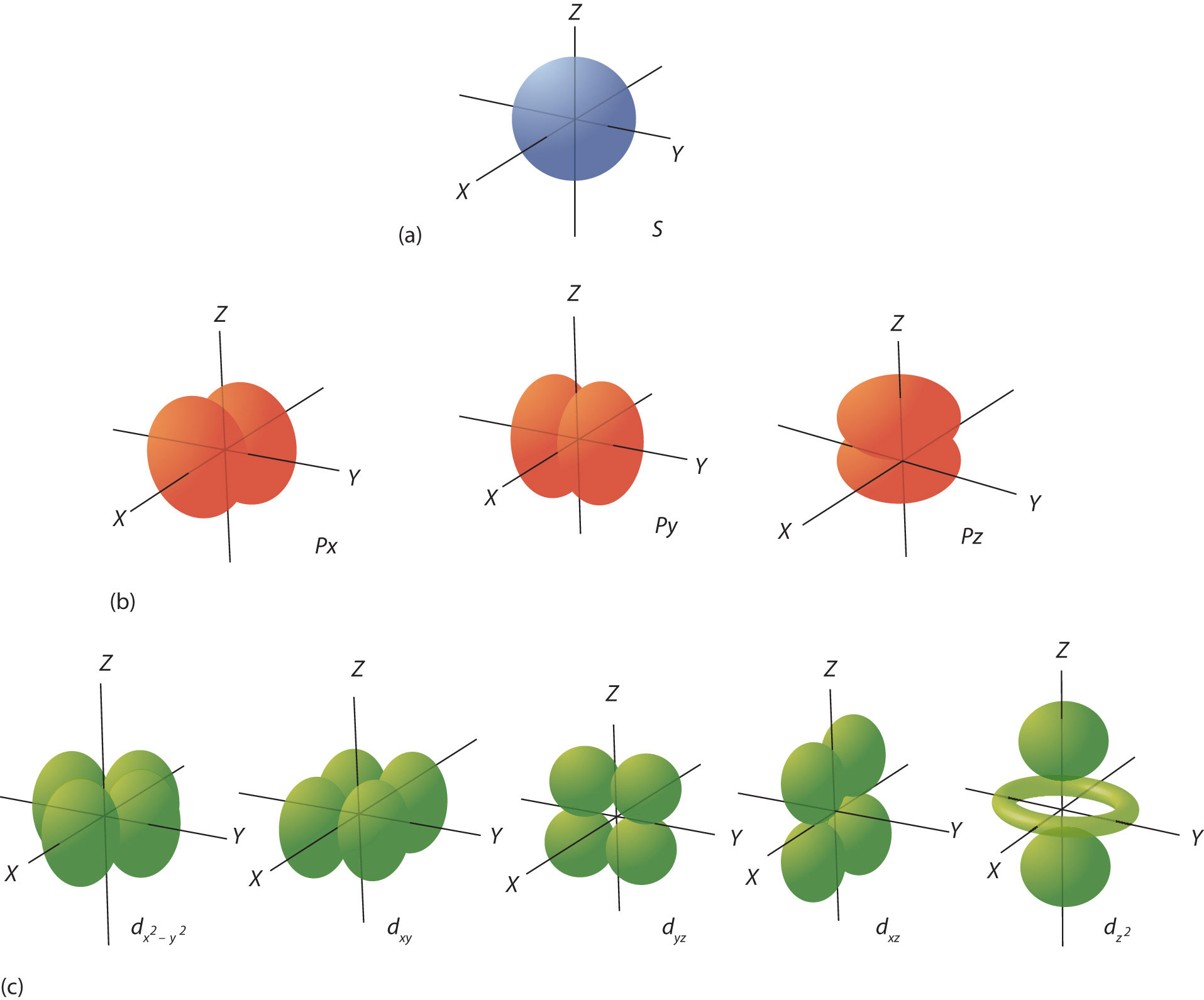
Figure showing s, p and d orbital shapes, reinforcing the number of orbitals in each sub-shell. Includes d orbitals, which extend beyond Periods 2 and 3 but support understanding of orbital structure. Source
These patterns explain why Periods 2 and 3 have eight elements each: 2 electrons in the s-sub-shell and 6 in the p-sub-shell.
Block Classification of the Periodic Table
The periodic table is divided into blocks based on the type of sub-shell that receives the highest-energy electron. This classification reinforces periodicity by grouping elements with similar electronic structures and therefore similar chemical behaviours.
Block Classification: The organisation of the periodic table into s-, p-, d- and f-blocks according to the sub-shell that receives the atom’s highest-energy electron.
The presence of this definition aligns with OCR expectations while supporting understanding of periodic arrangement.
The s-Block
The s-block includes Groups 1 and 2, plus hydrogen and helium. The distinguishing feature is that the highest-energy electron occupies an s-orbital:
Group 1 elements have an outer configuration ending in s¹.
Group 2 elements have an outer configuration ending in s².
These configurations explain the strong reactive trends in these groups, although the details of reactivity fall outside this subsubtopic.
The p-Block
The p-block spans Groups 13–18. Here the highest-energy electron occupies a p-orbital. Configurations end in p¹ to p⁶, depending on the group.
The p-block contains a wide range of element types, including:
Non-metals (e.g., oxygen, nitrogen)
Metalloids (e.g., silicon)
Some metals (e.g., aluminium)
These variations arise because p-orbitals allow greater diversity in bonding and structure.
The d-Block
The d-block contains the transition metals, which begin in Period 4. The highest-energy electron generally occupies a d-orbital, although the 4s orbital fills before the 3d orbital due to relative energy differences.
Even though the specification for this subsubtopic focuses on Periods 2 and 3, including the d-block in classification is essential, as OCR requires block terminology to be understood in full.
A normal sentence is included here to maintain spacing and clarity between structured sections.
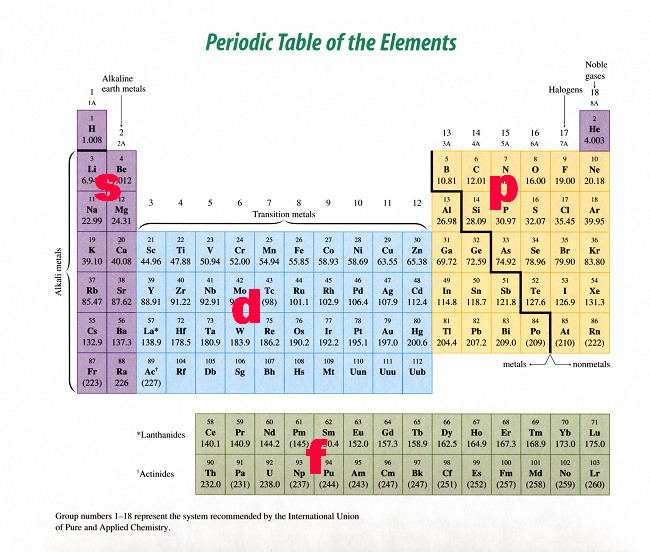
Periodic table showing s-, p-, d- and f-block regions, illustrating how highest-energy electrons define periodic table classification. Includes lanthanoids and actinoids, which extend beyond Periods 2 and 3. Source
Relationship Between Electron Configuration and Periodicity
Periodicity arises from recurring electron configuration patterns. As elements across a period fill equivalent sub-shell types, predictable trends emerge in:
Atomic radius
Ionisation energy
Electronegativity
Chemical reactivity
While detailed trends belong to other subsubtopics, their origin lies in the electron configuration patterns described here.
Key Observations
Elements with similar outer electron configurations appear in the same group.
Repetition of s- and p-sub-shell filling across adjacent periods creates repeating chemical behaviour.
Classification into s-, p-, and d-blocks provides a structural way to interpret these patterns.
Here is the corrected version using proper bullet points in plain text.
FAQ
Electron spin determines how electrons pair within orbitals. Each orbital holds two electrons with opposite spins, ensuring compliance with the Pauli Exclusion Principle.
Across a period, electrons first occupy empty degenerate orbitals singly with parallel spins before pairing occurs. This reduces electron–electron repulsion and stabilises the atom.
This behaviour supports the filling pattern of p-sub-shells in Periods 2 and 3.
The 4s orbital is slightly lower in energy than the 3d orbital in isolated atoms. Electrons always fill the lowest-energy orbital available first.
Once filled, the 3d orbitals can drop below 4s in energy for some transition metals, but this occurs after occupation. This subtle energy shift does not affect filling patterns in Periods 2 and 3.
Effective nuclear charge increases across a period, pulling electrons closer and slightly altering sub-shell energies.
This influences the relative positions of blocks:
s-block elements ionise most easily because their outer s-electrons experience lower effective nuclear charge.
p-block elements retain electrons more strongly due to increased effective nuclear charge across the period.
The block arrangement reflects these differences in electron behaviour.
The p-sub-shell contains three orbitals, allowing up to six electrons, creating more possible valence electron patterns than the two-electron s-sub-shell.
As a result, the p-block contains:
Metals, non-metals and metalloids
Elements with multiple oxidation states
A wide range of bonding types
This diversity arises from the variability in electron configurations within the p-sub-shell.
Block classification is based strictly on the sub-shell that receives the highest-energy electron in the ground-state electron configuration.
For borderline cases:
If the final electron enters an s-orbital, the element is s-block.
If it enters a p-orbital, it is p-block.
If it enters a d-orbital, it is d-block.
This rule applies consistently, even when orbital energies are very close.
Practice Questions
State what is meant by the term first ionisation energy. Explain why the first ionisation energy generally increases across a period from left to right.
(2 marks)
First ionisation energy: energy required to remove one mole of electrons from one mole of gaseous atoms. (1)
Increases across a period due to increasing nuclear charge with electrons added to the same shell, causing stronger attraction and higher energy required. (1)
Magnesium (Mg), aluminium (Al) and silicon (Si) are successive elements in Period 3 of the periodic table.
(a) Write the full electron configuration for Al.
(b) Aluminium is classified as a p-block element. Explain why, referring to electron configuration.
(c) Explain why Si has a higher first ionisation energy than Al, despite having a greater nuclear charge.
(5 marks)
(a)
Electron configuration of Al: 1s2 2s2 2p6 3s2 3p1 (or equivalent correct configuration). (1)
(b)
Highest-energy electron enters a p-sub-shell/p-orbital. (1)
Therefore Al is a p-block element. (1)
(c)
Si has a higher nuclear charge than Al. (1)
Both outer electrons are in the same shell, so shielding is similar. (1)
Greater nuclear attraction in Si means more energy is required to remove an electron. (1)

