OCR Specification focus:
‘First ionisation energy is removing one mole of electrons from one mole of gaseous atoms; trends explained by nuclear charge, atomic radius, shielding, and sub-shell effects.’
Introduction
First ionisation energy reveals how strongly atoms hold their outer electrons. Understanding its definition and periodic trends is essential for explaining reactivity and bonding patterns across the periodic table.
Definition of First Ionisation Energy
When studying periodicity, the term first ionisation energy is crucial because it reflects the energy required to remove an electron from an isolated atom in the gas phase.
First Ionisation Energy: The energy required to remove one mole of electrons from one mole of gaseous atoms to form one mole of gaseous 1+ ions.
Ionisation energies allow chemists to interpret atomic structure trends such as nuclear attraction, shielding, and subshell behaviour across a period or down a group.
Understanding the Ionisation Process
The Importance of the Gaseous State
Ionisation energy values are always measured for atoms in the gaseous state to ensure that all comparisons reflect intrinsic atomic attraction rather than interactions in liquids or solids.
The Ionisation Step
When an atom loses its outer electron, the following generalised process occurs:
Ionisation Step (IE₁) = X(g) → X⁺(g) + e⁻
X(g) = Gaseous atom
X⁺(g) = Gaseous singly charged positive ion
e⁻ = Electron removed
Removing this electron requires energy because the electron is attracted to the positive nucleus; the strength of this attraction dictates the magnitude of the ionisation energy.
A single sentence is placed here to ensure spacing before the next section.
Key Factors Affecting First Ionisation Energy
Ionisation energy depends on how strongly the nucleus attracts the outermost electron. The OCR specification emphasises nuclear charge, atomic radius, electron shielding, and sub-shell effects.
Nuclear Charge
A higher nuclear charge means more protons in the nucleus pulling electrons inward more strongly. Increasing nuclear charge generally increases first ionisation energy.
Atomic Radius
A larger atomic radius places outer electrons further from the nucleus, reducing electrostatic attraction and lowering first ionisation energy.
Electron Shielding
Increased shielding by inner-shell electrons reduces the effective nuclear attraction experienced by the outer electron, lowering the first ionisation energy.
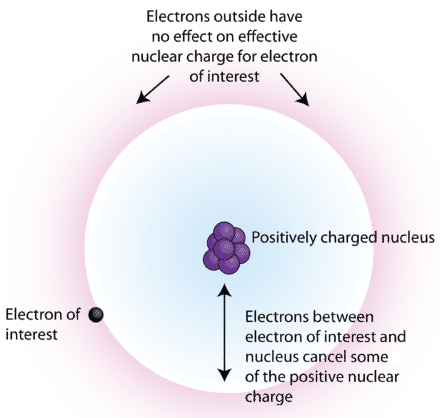
This schematic atom shows inner electrons forming a shielding cloud between the nucleus and an outer electron. The inner electrons partially cancel the positive charge felt by the outer electron. The figure is generic, but the shielding principle applies universally and underpins ionisation energy trends. Source
Sub-Shell Effects
Ionisation energy is also influenced by the distribution of electrons across s-, p-, and d-sub-shells.
Key sub-shell effects include:
s-electrons are slightly closer to the nucleus and experience stronger attraction, increasing ionisation energy relative to p-electrons in the same shell.
Electron pairing within orbitals increases repulsion, making paired electrons easier to remove.
Half-filled sub-shells (such as p³) offer extra stability, increasing ionisation energy.
A short sentence here ensures separation from the next content block.
General Periodic Trends in First Ionisation Energy
Trend Across a Period (e.g., Periods 2 and 3)
Across a period, first ionisation energy generally increases from left to right because nuclear charge increases while shielding is similar and atomic radius decreases.
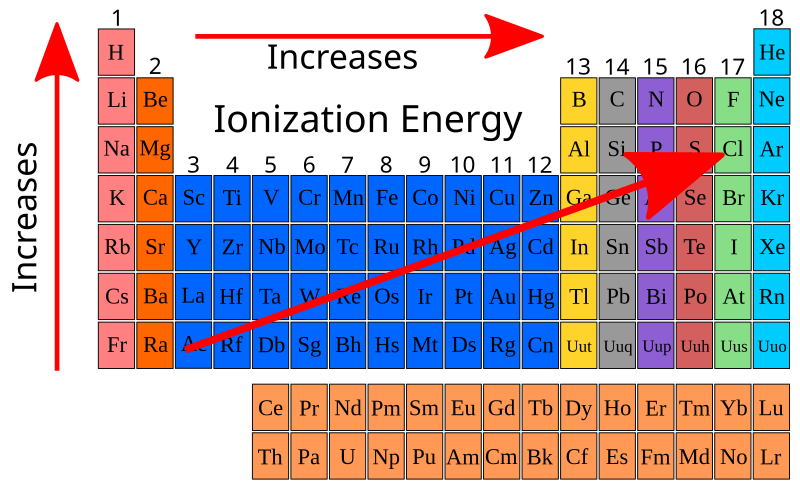
This periodic table highlights the trend of increasing first ionisation energy moving up groups and across periods. Noble gases show the highest values, while alkali metals show the lowest. The diagram includes all elements, extending beyond Periods 2 and 3, though the same principles apply. Source
However, there are notable exceptions due to sub-shell structure:
A drop occurs between Be → B (and Mg → Al) because the electron to be removed in B is from a higher-energy p-orbital.
Another drop occurs between N → O (and P → S) because O has a paired p-electron, which experiences repulsion and is more easily removed.
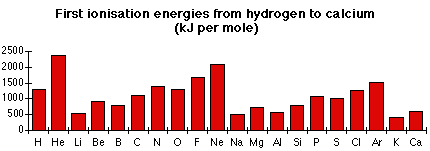
This graph plots first ionisation energies for elements H–Ca, illustrating periodicity through its repeating peaks. Small dips at boron and oxygen correspond to sub-shell and electron-pairing effects. The graph includes elements beyond Periods 2 and 3, but the same principles apply. Source
Trend Down a Group
Moving down a group:
Atomic radius increases significantly as new electron shells are added.
Shielding increases, reducing the effective nuclear attraction.
Although nuclear charge rises, the increased distance and shielding outweigh it.
Thus, first ionisation energy decreases down a group, meaning atoms lose outer electrons more easily.
A linking sentence is included here before presenting the next section.
Explaining the Trends Using the OCR Specification Factors
1. Nuclear Charge
Higher nuclear charge increases attraction between the nucleus and outer electrons, raising ionisation energy. Across periods, nuclear charge strengthens the trend of increasing energy.
2. Atomic Radius
As radius increases, ionisation energy decreases because the outer electron is further from the nucleus and less strongly attracted. This principle is essential when comparing groups.
3. Electron Shielding
Greater shielding by filled inner shells reduces the effective nuclear charge felt by outer electrons. Down groups, shielding increases strongly, causing clear decreases in ionisation energies.
4. Sub-Shell Effects
Sub-shell structure explains deviations from smooth periodic trends:
p-electrons are removed more easily than s-electrons in the same shell.
Paired electrons require less energy to remove due to electron-electron repulsion.
A single sentence here separates the final section.
Why First Ionisation Energy Matters in Periodicity
Understanding first ionisation energy supports analysis of:
Element reactivity, especially within groups.
Metallic vs non-metallic behaviour across periods.
Bond formation, as elements with low first ionisation energies form cations readily.
Periodic trends, including prediction of unknown element behaviour.
These principles directly reflect the OCR focus on the relationship between first ionisation energy, atomic structure, and trends across the periodic table.
FAQ
Ionisation energies must be determined using gaseous atoms because this state avoids intermolecular forces that exist in liquids and solids. These forces would distort the true energy required to remove an electron.
In the gas phase, each atom behaves independently, meaning the measured value reflects only the attraction between the nucleus and the electron being removed.
Successive ionisation energies show the increasing difficulty of removing electrons as remaining electrons experience greater effective nuclear charge.
A large jump between two successive energies indicates removal from a new, inner shell that is much closer to the nucleus. Although this does not change the first ionisation energy itself, it helps explain electron arrangement and nuclear attraction patterns.
Nuclear charge refers to the total number of protons in the nucleus.
Effective nuclear charge is the net attractive force acting on an outer electron after accounting for shielding by inner electrons.
This distinction matters because two elements with similar proton numbers may still have different first ionisation energies if their shielding differs.
Noble gases have complete outer shells, making them electronically stable and tightly bound.
They also possess small atomic radii within their periods, placing outer electrons close to the nucleus.
Combined, these factors mean that a large amount of energy is required to remove an electron from a noble gas atom.
Transition metals introduce electrons into d-sub-shells, which alters the pattern of shielding and nuclear attraction.
d-electrons shield outer electrons less effectively than s- and p-electrons, creating irregularities in the expected left-to-right increase.
As a result, ionisation energies across the transition metals fluctuate rather than rising steadily as they do in Periods 2 and 3.
Practice Questions
Explain why the first ionisation energy of oxygen is lower than that of nitrogen.
(2 marks)
1 mark: Electron removed from oxygen is from a paired p-orbital / paired electrons in oxygen cause repulsion.
1 mark: Repulsion makes the electron easier to remove, lowering the first ionisation energy.
The first ionisation energies of elements across Period 3 show a general increase, but with notable decreases at aluminium and sulfur.
Using your knowledge of atomic structure, explain:
(a) Why the general trend increases across the period.
(b) Why aluminium has a lower first ionisation energy than magnesium.
(c) Why sulfur has a lower first ionisation energy than phosphorus.
(5 marks)
(a) General increase across the period
1 mark: Nuclear charge increases across Period 3.
1 mark: Atomic radius decreases / electrons held more strongly (shielding remains similar).
(b) Al < Mg
1 mark: Aluminium’s outer electron is in a higher-energy p-orbital, while magnesium’s is in an s-orbital.
1 mark: The p-orbital electron is easier to remove, so aluminium has a lower first ionisation energy.
(c) S < P
1 mark: Sulfur has paired electrons in a p-orbital, causing electron-electron repulsion.
1 mark: Repulsion makes the paired electron easier to remove, lowering sulfur’s first ionisation energy.

