OCR Specification focus:
‘Elements arranged by increasing proton number in periods showing repeating trends and in groups with similar chemical properties (periodicity).’
The periodic table arranges elements in a structured pattern that reflects repeating chemical trends, enabling predictions about properties and behaviours across different periods and groups.
Structure of the Periodic Table
The Modern Periodic Table
The modern periodic table is organised according to increasing proton number, also referred to as atomic number. This arrangement ensures that elements follow a consistent pattern in their electronic structure, which directly influences their chemical behaviour. The table consists of periods (horizontal rows) and groups (vertical columns), each revealing specific and predictable periodic trends.
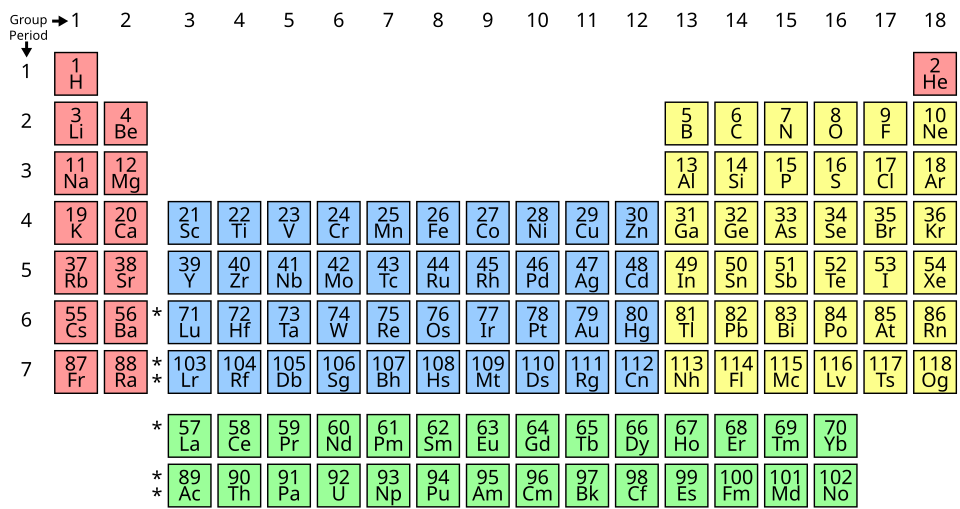
Periodic table arranged by increasing proton number, with each element in its correct period and group. Colours show the s-, d- and p-blocks, emphasising how electrons fill different regions. The block colouring adds slight extra detail but reinforces structural links to electron configuration. Source
Proton number: The number of protons in the nucleus of an atom; it defines the identity of an element.
After arranging elements in order of proton number, repeating patterns in chemical and physical properties become evident. These repeating patterns are known as periodicity, a central idea in understanding the periodic table and predicting the behaviour of unfamiliar elements.
Periods and Their Significance
A period is a horizontal row on the periodic table. Periods indicate a progression in the filling of electron shells as proton number increases.
Period: A horizontal row of elements in the periodic table showing a progression in electron shell filling.
Across a period, elements gradually change from metallic to non-metallic character. This pattern reflects changes in nuclear charge, atomic radius, and electron configuration. Although properties vary across a period, they do so systematically, which is essential for anticipating reactivity and bonding tendencies.
Groups and Chemical Similarity
A group is a vertical column of elements that share similar chemical properties because they possess the same number of electrons in their outer shell.
Group: A vertical column of elements with similar chemical properties due to a shared number of outer-shell electrons.
Group trends arise because valence electrons determine most chemical reactions. For example, Group 1 metals all form 1+ ions, whereas Group 17 elements (halogens) each form 1– ions when gaining an electron. These similarities lead to comparable patterns in reactivity, bonding, and compound formation.
A brief sentence ensures separation before further structured information.
Understanding Periodicity
Periodicity refers to the repeating trends in physical and chemical properties observed across periods. Because elements are arranged by increasing proton number, their outer electron configurations follow a periodic pattern. This leads to recurring trends such as:
Atomic radius decreasing across a period.
Ionisation energy increasing across a period.
Electronegativity increasing from left to right.
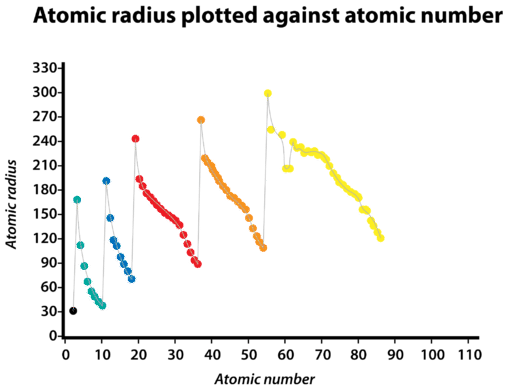
Graph showing atomic radius plotted against atomic number, with peaks and troughs illustrating periodicity across periods. Each period is coloured differently to highlight repeating patterns. The numerical detail exceeds syllabus expectations but effectively visualises periodic trends. Source
These trends arise due to increased nuclear charge and reduced shielding effects as electrons are added to the same shell. Periodicity forms the basis for predicting element behaviour, especially when encountering unfamiliar substances.
Electron Shells and Sub-shell Patterns
The structure of the periodic table reflects the organisation of electrons into shells and sub-shells. As proton number increases, electrons fill available spaces according to established rules. Period number corresponds to the highest occupied electron shell for the ground state of an element.
Key ideas include:
Elements in the same period have electrons occupying the same number of shells.
Elements in the same group share the same number of outer-shell electrons.
Changes in sub-shell occupation across a period explain trends in reactivity and bonding.
Relationship Between Structure and Chemical Behaviour
The periodic table’s layout is not random; every position has meaning. Because electron arrangement governs chemical behaviour, the periodic structure enables prediction. Important relationships include:
Elements on the left of the table tend to be metals, with low ionisation energies and a tendency to form positive ions.
Elements on the right of the table tend to be non-metals, with high electron affinities and a tendency to gain electrons.
Noble gases at the far right of the table have full outer shells, resulting in minimal chemical reactivity.
These overarching patterns create a systematic framework used widely in organic, inorganic, and physical chemistry.
Block Structure of the Periodic Table
Although block classification is covered more deeply in another subtopic, it is essential here to understand that the table can be divided based on sub-shell filling:
s-block (Groups 1–2)
p-block (Groups 13–18)
d-block (transition metals)
This division reinforces periodicity by reflecting the sequences of electron filling and the resulting similarities in physical and chemical properties.
A follow-up sentence maintains clarity before moving to the next point.
Why Increasing Proton Number Matters
Increasing proton number is fundamental to the periodic table’s structure because it leads to predictable changes in electron configuration. As elements are ordered in this way:
Trends such as metallic to non-metallic character across a period become clear.
Patterns in reactivity, melting point, and ion formation can be logically interpreted.
Elements within a group display consistent ionic charges and bonding behaviour.
The periodic table’s design enables chemists to infer the properties of elements not previously studied, demonstrating why periodicity is one of the most powerful tools for organising chemical knowledge.
FAQ
The current structure originates from Mendeleev’s early table, which arranged elements by atomic mass. Later discoveries about subatomic particles showed that atomic number, not mass, determines an element’s identity.
When proton number replaced atomic mass as the organising principle, inconsistencies in Mendeleev’s arrangement were resolved, creating the modern layout used today.
Period length depends on how many electrons can occupy the available sub-shells in each energy level.
For example:
Period 2 contains 8 elements because the 2s and 2p sub-shells hold a total of 8 electrons.
Period 3 also holds 8 for the same reason.
Period 4 is much longer due to the addition of 3d sub-shell filling.
Period lengths therefore reflect electron capacity, not chemical similarity.
The boundary reflects a gradual change in electronic behaviour as nuclear charge increases across a period.
Metals on the left lose electrons easily due to lower attraction between the nucleus and outer electrons.
Non-metals on the right gain electrons more readily because of stronger nuclear attraction.
The stepped line is a visual guide, and some elements adjacent to it (metalloids) show mixed metallic and non-metallic characteristics.
Noble gases form Group 18 because they all have full outer electron shells.
Their position highlights an important structural pattern:
Each period ends with a noble gas.
Full outer shells act as the “reset point” before the next period begins.
The group placement emphasises the periodic cycle of electron configuration rather than chemical reactivity.
Bonding trends shift systematically as metallic character decreases and non-metallic character increases.
Across a period:
Metals on the left tend to form metallic bonds.
Metalloids in the middle may form covalent networks or variable bonding types.
Non-metals on the right typically form covalent bonds.
These bonding changes correspond directly to movement across the table and increasing attraction between nucleus and electrons.
Practice Questions
Explain why elements in the same group of the periodic table have similar chemical properties.
(2 marks)
Elements in the same group have the same number of electrons in their outer shell. (1 mark)
This results in similar chemical reactions or similar chemical behaviour. (1 mark)
The modern periodic table is arranged by increasing proton number.
(a) Describe what is meant by the term proton number. (1 mark)
(b) Explain how arranging elements in order of increasing proton number leads to periodicity in their chemical properties. (4 marks)
(5 marks)
(a)
Proton number is the number of protons in the nucleus of an atom. (1 mark)
(b)
Award up to 4 marks for the following points:
Increasing proton number increases the number of electrons in a predictable pattern. (1 mark)
This leads to recurring outer electron configurations across periods. (1 mark)
Outer electron configuration determines chemical behaviour. (1 mark)
Therefore similar patterns in properties repeat at regular intervals, producing periodicity. (1 mark)

