OCR Specification focus:
‘Activation energy is the minimum energy required for reaction, illustrated using enthalpy profile diagrams and related to rate.’
Understanding activation energy is essential for explaining why reactions occur at different speeds. This topic explores how energy profiles represent reaction pathways and how activation energy influences reaction rate.
Activation Energy: Core Concepts
Chemical reactions require particles to collide with sufficient energy to form products. This minimum energy is known as activation energy, and it determines whether collisions are successful. Activation energy is central to understanding why some reactions proceed rapidly while others occur slowly or only under certain conditions such as heating.
Defining Activation Energy
When reactants approach one another, they must overcome an energy barrier associated with bond breaking and rearrangement before new bonds form. Introducing the formal terminology at this stage provides clarity for interpreting enthalpy profile diagrams.
Activation Energy: The minimum energy that reacting particles must possess for a reaction to occur.
This threshold energy is linked directly to the reaction’s rate: higher activation energies generally lead to slower reactions because fewer molecules have sufficient energy to react at a given temperature.
Energy Profiles and Reaction Pathways
Energy profiles (also known as enthalpy profile diagrams) illustrate how the enthalpy of a system changes during a reaction. These diagrams compare the energy of reactants and products, showing the activation energy barrier and the overall enthalpy change. They help visualise the route a reaction follows from reactants to products.
Components of an Energy Profile
A typical energy profile includes the following clearly identifiable features:
Reactant energy level
Represents the starting enthalpy of the system.Product energy level
Represents the final enthalpy after the reaction.Activation energy peak
The highest point on the curve, showing the required energy barrier.Transition state
A short-lived, high-energy configuration where old bonds begin to break and new bonds begin to form.ΔH (enthalpy change)
The difference between product and reactant energy levels.
The transition state represents the moment at which the system has maximum potential energy, and the arrangement of atoms is unstable. Small changes in temperature can dramatically affect the formation of this state because they influence the proportion of molecules with enough energy to reach it.
Normal sentence between definition and equation blocks.
Arrhenius Relationship (k) = A e^(–Ea/RT)
k = Rate constant (units vary with reaction order)
A = Frequency factor (s⁻¹), representing collision frequency and orientation suitability
Ea = Activation energy (J mol⁻¹)
R = Gas constant (8.314 J mol⁻¹ K⁻¹)
T = Temperature (K)
Although full mathematical treatment is not required at this stage, recognising that the rate constant depends on activation energy and temperature reinforces the conceptual link with reaction rate.
Exothermic and Endothermic Profiles
While this subsubtopic does not focus on enthalpy change itself, recognising how activation energy appears in both exothermic and endothermic processes strengthens understanding of energy diagrams.
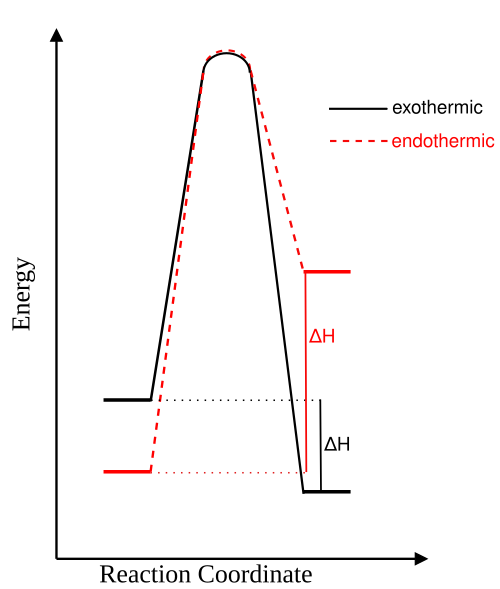
Energy profile diagrams comparing exothermic and endothermic reactions. Each curve shows reactant and product energy levels, activation energy, and enthalpy change ΔH. Extra detail includes side-by-side comparison for clearer contrast. Source
Exothermic Reactions
In exothermic reactions, products have lower enthalpy than reactants. An energy profile for such a reaction shows:
A downward overall enthalpy change (negative ΔH).
A distinct activation energy peak above the reactant level.
The release of energy as the reaction proceeds.
Even though these reactions release energy, they still require activation energy to begin because existing bonds must be broken before the reaction can progress.
Endothermic Reactions
Endothermic reactions require continuous energy input because:
Products have higher enthalpy than reactants.
The activation energy barrier is often larger than in exothermic reactions.
Energy is taken in from the surroundings as the reaction proceeds.
A clear understanding of both diagram types helps students compare reactions and identify the features that determine whether a process absorbs or releases heat.
Activation Energy and Reaction Rate
The link between activation energy and rate is fundamental in physical chemistry. Reaction rate depends on the number of effective collisions per unit time. If only a small fraction of molecules possess energy equal to or greater than the activation energy, the reaction proceeds slowly. Increasing temperature gives more particles the energy required to overcome this barrier, significantly increasing rate.
Key Factors Affecting Rate Through Activation Energy
Students should recognise the following factors that affect rate via activation energy:
Temperature
Raising temperature increases the proportion of molecules with energy exceeding the activation energy threshold, accelerating the reaction.Catalysts (not discussed in detail here)
Although studied in later subtopics, catalysts lower the activation energy by providing an alternative pathway with a reduced energy barrier.Bond strength
Stronger bonds require more energy to break, increasing the activation energy for the reaction.
These conceptual points help illustrate why activation energy is central to understanding chemical kinetics.
Visualising Activation Energy on Energy Profiles
Energy profile diagrams allow comparison of reaction pathways by clearly representing:
The initial energy of reactants
The activation energy peak
The energy of products
The enthalpy change
Such diagrams are indispensable for explaining how activation energy influences both reaction feasibility and reaction rate. They reinforce the relationship between energy changes and particle behaviour, showing how molecular energy distribution shapes chemical processes.
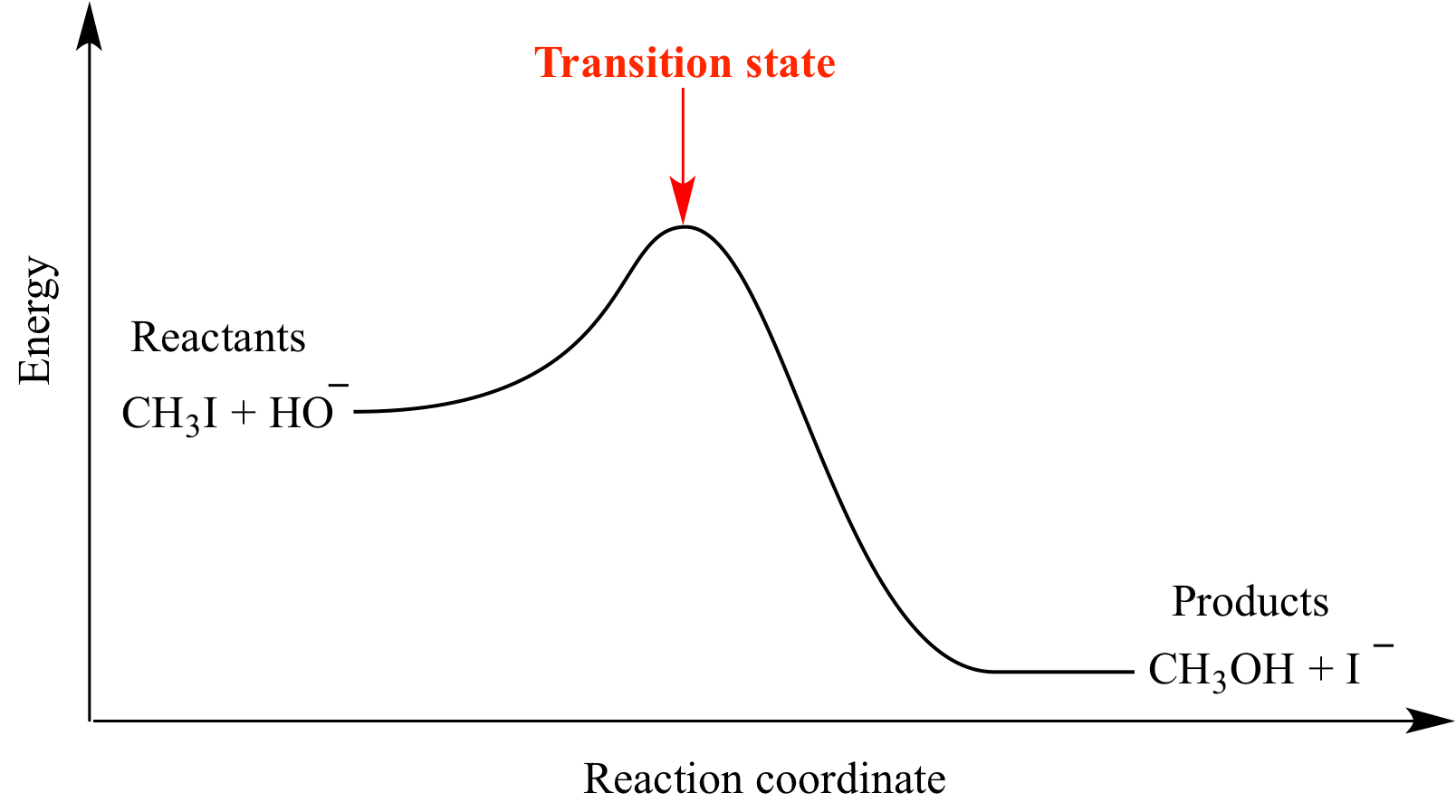
Energy profile diagram for an SN2 reaction showing reactants, a high-energy transition state, and products. The activation energy corresponds to the height of the peak above reactants. Specific reactants shown are additional detail beyond syllabus requirements. Source
Here are the same questions and mark schemes, but with standard text bullet points instead of the “•” symbol.
FAQ
Activation energy determines the minimum amount of energy particles must have to react. If the temperature is too low for enough particles to meet this threshold, the reaction may be effectively non-feasible under those conditions.
A reaction with a very high activation energy may only become feasible when heated significantly, even if the overall enthalpy change is favourable.
Activation energy depends not only on bond strength but also on the mechanism of the reaction and the arrangement of atoms during the transition state.
Small structural differences can alter how reactants must reorient or distort, leading to different energy barriers even for reactions involving comparable bonds.
The transition state is unstable because bonds are partially broken and partially formed, placing atoms in positions they do not normally occupy.
This arrangement creates significant electron repulsion or stretching of bonds, both of which contribute to the elevated energy required to reach this point.
Impurities, surface effects, or solvent interactions can modify how reactant particles collide, altering the effective energy barrier.
For example, polar solvents may stabilise certain transition states, lowering the activation energy, while impurities on solid surfaces can either promote or hinder the formation of activated complexes.
The activation energy for each direction is measured from the respective reactant energy level to the transition state. If products lie higher or lower in energy than reactants, the distance to the peak changes.
This means:
Exothermic reactions have a larger reverse activation energy.
Endothermic reactions have a larger forward activation energy.
Practice Questions
State what is meant by the term activation energy and explain how temperature affects the proportion of molecules able to react.
(2 marks)
Activation energy is the minimum energy required for particles to react. (1)
Increasing temperature increases the proportion of molecules with energy equal to or greater than the activation energy. (1)
The diagram below represents an energy profile for a reaction.
Reactants → peak → products
Using ideas about activation energy and reaction pathways:
a) Describe the meaning of the peak shown on the energy profile.
b) Explain why a catalyst increases the rate of reaction but does not change the overall enthalpy change.
c) Discuss why reactions with high activation energies are often slow at room temperature.
(5 marks)
a)
The peak represents the transition state or activated complex. (1)
It is the point of maximum energy that reactants must reach before forming products. (1)
b)
A catalyst provides an alternative reaction pathway with a lower activation energy. (1)
The catalyst does not affect the energy levels of reactants or products, so the enthalpy change remains the same. (1)
c)
At room temperature only a small fraction of molecules have sufficient energy to overcome a large activation energy barrier. (1)

