OCR Specification focus:
‘Apply Hess’ law using combustion or formation data to determine enthalpy changes indirectly with appropriate enthalpy cycles; outline required techniques.’
Hess’ law and enthalpy cycles allow chemists to determine enthalpy changes that cannot be measured directly, using indirect routes based on formation or combustion data.
Hess’ Law: Core Ideas
Hess’ law is central to indirect enthalpy calculations in thermochemistry. It relies on the principle that enthalpy is a state function, meaning the overall enthalpy change depends only on the initial and final states, not the path taken. This makes it possible to calculate enthalpy changes using alternative chemical routes when direct measurement is impractical.
Hess’ law: The enthalpy change of a reaction is the same regardless of the route taken, provided the initial and final conditions are identical.
In practice, Hess’ law is applied using enthalpy cycles, which visually map out the different pathways connecting the same reactants and products. These cycles often use standard enthalpy changes of formation or standard enthalpy changes of combustion, depending on the data available.
Standard Enthalpy Changes in Cycles
Before applying Hess’ law, it is important to understand the enthalpy values commonly used in cycles.
Standard enthalpy of formation: The enthalpy change when one mole of a compound is formed from its elements in their standard states under standard conditions.
A sentence ensures separation before the next definition block is introduced.
Standard enthalpy of combustion: The enthalpy change when one mole of a substance reacts completely with oxygen under standard conditions.
These values allow chemists to build indirect pathways, enabling the determination of enthalpy changes such as enthalpy of reaction, which may be difficult to obtain experimentally.
Constructing Enthalpy Cycles
Enthalpy cycles organise information so that unknown enthalpy changes can be deduced mathematically. They must show:
The target reaction with its unknown enthalpy change.
The alternative routes using known ΔHf or ΔHc values.
Arrows indicating direction, with consistent orientation throughout the cycle.
When constructing a cycle based on formation data, all species must be expressed relative to their constituent elements in standard states. When constructing one using combustion data, all substances are shown reacting with oxygen to form typical combustion products such as CO₂ and H₂O.
Formation Cycles
Formation cycles are often used when the specification provides formation enthalpies. The key principle is that:
Reactants → Elements → Products
Each arrow has an associated enthalpy change.
The indirect route through elements must equal the enthalpy of the direct reaction.
In a formation enthalpy cycle, both reactants and products are linked by arrows from a base line representing their elements in standard states.
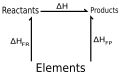
This triangular enthalpy cycle shows reactants and products at the top, both connected to the elements in their standard states at the base. The arrows are labelled with enthalpy changes for formation and for the overall reaction, illustrating that the sum of the stepwise enthalpy changes equals the direct route. This visual reinforces that Hess’ law allows ΔH to be found from formation data, regardless of the path taken. Source
Combustion Cycles
Combustion cycles frequently appear in questions involving organic compounds, where combustion enthalpies are widely available. Their structure typically follows:
Reactants → Combustion products
Products → Combustion products
Because both sides combust to the same final substances, Hess’ law allows the enthalpy change of reaction to be deduced by comparing these pathways.
Mathematical Treatment of Cycles
Although no worked examples are included here, students must understand that enthalpy cycles rely on simple algebra. The direction of arrows is crucial:
Arrows with the direction of the cycle are added as written.
Arrows against the direction are subtracted.
Hess’ Law Relationship (ΔHreaction) = Σ(ΔHroute 1) − Σ(ΔHroute 2)
ΔHreaction = Enthalpy change for the reaction (kJ mol⁻¹)
ΔHroute 1/ΔHroute 2 = Sum of enthalpy changes of alternative routes (kJ mol⁻¹)
This expression illustrates the general mathematical structure used to combine enthalpy values, though the exact form varies depending on the type of cycle.
Choosing Between Formation and Combustion Data
Students should select the type of enthalpy cycle that aligns with the data supplied:
Use formation cycles when ΔHf values are available for all reactants and products.
Use combustion cycles when ΔHc values are provided, especially for hydrocarbons or organic molecules.
Important terminology in this area includes state symbols, standard conditions, and consistent stoichiometry, all of which must match the data provided in the question.
Techniques for Applying Hess’ Law
To correctly apply Hess’ law in an exam or practical calculation context, students should follow a structured approach:
Identify the target enthalpy change required.
Rewrite all equations so that the substances appear in the correct form and state.
Ensure all chemical equations are balanced.
Construct the cycle using arrows pointing in consistent directions.
Apply the Hess’ law relationship, keeping careful track of sign conventions.
Key Considerations
Combustion products are typically CO₂(g) and H₂O(l) unless otherwise stated.
For formation cycles, all elements must be written in their standard states (e.g., O₂(g), H₂(g), C(s, graphite)).
The accuracy of the cycle depends on correctly interpreting and arranging the given data.
The sign of ΔH is essential: combustion enthalpies are usually negative, formation values may be positive or negative.
Hess’ law shows that the enthalpy change between reactants and products is the same whether the reaction occurs in one step or via several intermediate steps.
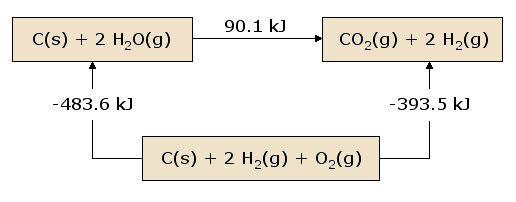
This diagram shows reactants and products in separate boxes, connected directly and via an intermediate box representing an alternative reaction pathway. Each arrow is labelled with an enthalpy change, demonstrating that the sum of enthalpy changes along the multi-step route equals the enthalpy change for the single-step route. The specific reaction and ΔH values shown exceed syllabus requirements but serve as a clear illustration of Hess’s law. Source
Why Hess’ Law Is Essential in Thermochemistry
Hess’ law is a fundamental tool because many enthalpy changes cannot be measured directly. For instance, reactions involving unstable intermediates, highly exothermic steps, or slow kinetics may not be experimentally viable. Enthalpy cycles allow these values to be calculated using measurable data, providing essential insights into reaction energetics and supporting predictions of feasibility and thermodynamic behaviour.
FAQ
Chemists choose the data type based on which enthalpy values are available for all substances in the reaction. Formation data are preferred when reliable values exist for every reactant and product.
Combustion data are often used for organic compounds because their combustion enthalpies are widely tabulated and highly consistent.
Arrow direction represents the physical direction of the enthalpy change. If an arrow is reversed relative to the desired pathway, the sign of the enthalpy value must be changed.
This ensures the mathematical relationship in Hess’ law correctly reflects whether the enthalpy change is being followed or opposed.
Cycles using formation data require elements because formation enthalpies relate every substance back to its constituent elements, providing a universal reference point.
Combustion cycles skip elemental references because they rely on all substances combusting to a shared set of products.
Typical errors include:
Incorrect balancing of equations.
Not matching physical states (such as liquid water vs. gaseous water).
Using coefficients that do not match the enthalpy data provided.
These mistakes lead to incorrect enthalpy calculations even if the cycle structure is correct.
If a required value is missing, chemists may:
Use tabulated alternative data (e.g., standard enthalpy of atomisation).
Rearrange known equations to generate the missing enthalpy indirectly.
Hess’ law works as long as all steps describe valid thermodynamic pathways connecting the same start and end points.
Practice Questions
State Hess’ law and explain why it allows chemists to determine enthalpy changes indirectly. (2 marks)
States that the enthalpy change of a reaction is independent of the route taken, provided initial and final conditions are the same. (1 mark)
Explains that this allows indirect calculation because alternative pathways can be used when direct measurement is not possible. (1 mark)
A student uses standard enthalpies of combustion to determine the enthalpy change for a reaction.
Explain how a combustion enthalpy cycle would be constructed and how it would be used to calculate the reaction enthalpy.
Your answer should refer to arrows, direction, and the products formed in combustion.
(5 marks)
States that all reactants and products are shown combusting to the same combustion products, typically carbon dioxide and water. (1 mark)
Describes drawing arrows from reactants to combustion products with their enthalpies of combustion. (1 mark)
Describes drawing arrows from products to combustion products with their enthalpies of combustion. (1 mark)
States that Hess’ law is applied by comparing the indirect and direct routes. (1 mark)
Correctly explains that arrows followed in the opposite direction to the cycle must be subtracted when calculating the reaction enthalpy. (1 mark)

