OCR Specification focus:
‘Average bond enthalpy is energy to break one mole of gaseous bonds; use bond energies to estimate reaction enthalpies and explain bond breaking and making.’
Average bond enthalpies allow chemists to estimate enthalpy changes by considering the energy required to break and form bonds during reactions, offering a valuable approach when experimental data are unavailable.
Average Bond Enthalpies
Average bond enthalpies are central to understanding how energy is absorbed and released during chemical reactions. They provide a useful method for estimating enthalpy changes when direct experimental measurements are impractical or incomplete.
Definition of Average Bond Enthalpy
When discussing energy changes in reactions, the term average bond enthalpy appears frequently because it highlights the energetic cost of breaking chemical bonds.
Average Bond Enthalpy: The energy required to break one mole of a specified type of bond in the gaseous state, averaged across many similar compounds.
Average bond enthalpies reflect mean values because a given bond can vary slightly in strength depending on molecular environment. This makes them valuable for estimation but not for obtaining precise thermodynamic values. The fact that they always refer to the gaseous state ensures consistency across data sources.
Bond Breaking and Bond Making
Chemical reactions involve both breaking bonds in reactants and forming new bonds in products. These processes underpin the energy changes observed.
Bond Breaking: An endothermic process in which energy is absorbed to separate atoms in a chemical bond.
A sentence explaining this follows: breaking bonds always requires energy input because atoms must overcome attractive forces holding them together.
Bond Making: An exothermic process in which energy is released when new chemical bonds form between atoms.
Energy changes during reactions arise from the balance between these two processes. A reaction will be exothermic overall if more energy is released during bond formation than is required for bond breaking. Conversely, if more energy is absorbed than released, the reaction is endothermic. This links directly to the enthalpy change of reaction, which can be estimated using bond enthalpy data.
Calculating Enthalpy Changes Using Average Bond Enthalpies
Bond enthalpy calculations rely on a structured approach to account for the energy changes associated with each bond broken and formed. Because average bond enthalpies are always positive values (energy input), careful attention must be given to signs when estimating ΔH for reactions.
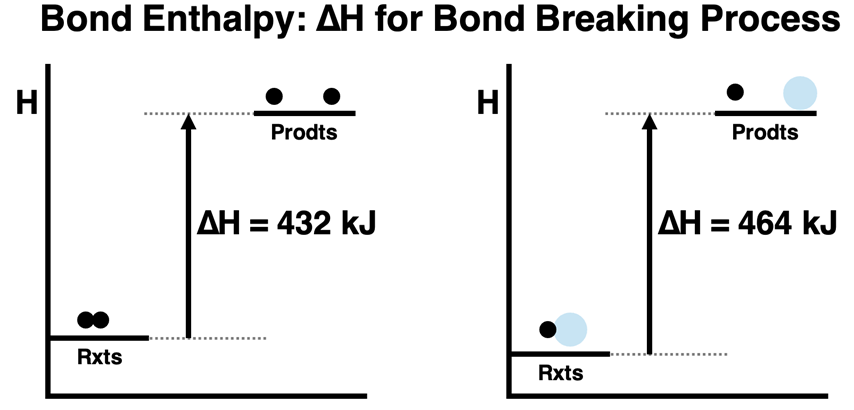
This diagram shows enthalpy-level diagrams for breaking H–H and H–O bonds, with vertical arrows indicating the energy absorbed. It highlights that bond enthalpy represents the energy required to break one mole of gaseous bonds. The inclusion of two different bond types adds detail beyond the syllabus but reinforces differences in bond strength. Source
Process for Estimating ΔH
To estimate an enthalpy change using average bond enthalpies, chemists follow a consistent set of steps. These reflect the conceptual view that reactions proceed via breaking all reactant bonds before forming all product bonds.
Identify all bonds broken in the reactants.
Identify all bonds formed in the products.
Use the relationship:
Energy in (bond breaking) is endothermic.
Energy out (bond forming) is exothermic.
Sum energies for all bonds broken and all bonds formed separately.
Combine the two totals to estimate overall reaction enthalpy.
Key Equation Format
The OCR specification requires students to understand how the energetic balance leads to an estimated reaction enthalpy. This is represented using the following equation.
Estimated Enthalpy Change (ΔH) = Σ(Bond Enthalpies of Bonds Broken) − Σ(Bond Enthalpies of Bonds Formed)
ΔH = Enthalpy change of reaction, measured in kJ mol⁻¹
Bond Enthalpy = Energy required to break one mole of gaseous bonds, measured in kJ mol⁻¹
Between these calculations, it is essential to remember that because bond enthalpies are average values, they may not exactly match enthalpy changes determined through experimental methods such as calorimetry. However, they remain highly useful for predicting trends and estimating energy changes to a good approximation.
Why Average Bond Enthalpies Differ from Experimental Data
Bond enthalpies are averaged from many compounds, making them approximate values. Experimental enthalpy changes depend on actual molecular environments, phases, and conditions. For example, enthalpy changes of formation and combustion refer to standard conditions and standard states, whereas average bond enthalpies always refer to gaseous species. This explains why calculated values using bond enthalpies often differ from experimental data, yet remain consistent with theoretical expectations.
Factors Influencing Bond Enthalpy Values
Bond enthalpies vary with several molecular features, which students must understand clearly.
Molecular Environment
Even identical types of bonds (e.g., O–H, C–H) can vary in strength depending on molecular geometry, electronegativity differences, and the presence of adjacent atoms or functional groups.
Multiple Bonds
Multiple bonds generally possess larger bond enthalpies than single bonds because they involve higher electron density and stronger attractions between atoms.
Double bonds have higher bond enthalpies than single bonds.
Triple bonds have the highest bond enthalpies for a given pair of atoms.
Bond Polarity and Covalent Character
Bonds involving significant electronegativity differences often require more energy to break. Increased polarity or partial ionic character typically strengthens a bond, influencing the value of its bond enthalpy.
Application in Organic and Inorganic Chemistry
Average bond enthalpies are extensively used across organic and inorganic systems.
In organic chemistry, they help estimate enthalpy changes for reactions such as substitution, addition, or combustion.
In inorganic chemistry, they support understanding of simple gas-phase reactions and provide insight into trends across the periodic table.
These notes align with the OCR requirement to use bond energies to estimate reaction enthalpies and explain the roles of bond breaking and making, forming a foundational skill in thermochemistry.
This figure compares exothermic and endothermic reactions through simple energy-level diagrams. Reactant and product enthalpy levels are shown with arrows indicating energy release or absorption. The diagrams include general thermochemical information beyond the syllabus but directly reinforce how bond enthalpy differences relate to the sign of ΔH. Source
FAQ
Average bond enthalpies are compiled from many experimental measurements, and different datasets may use slightly different reference molecules, temperature conditions, or statistical averaging methods.
Some tables prioritise gas-phase spectroscopic measurements, while others rely on thermochemical cycles.
Variations are most pronounced for bonds in molecules with strong resonance or extensive delocalisation.
The method assumes all bonds of the same type have identical strength, which ignores molecular context.
In multi-step mechanisms, transition states and intermediate species may involve partial bonds or distorted structures, making simple bond counting inaccurate.
They provide good estimates but lack the precision required for detailed mechanistic enthalpy analysis.
Resonance spreads electron density over several atoms, meaning individual bonds do not match the strength implied by a standard single or double bond.
For example, the C–O bonds in carboxylates or the C–C bonds in benzene have intermediate strengths.
This makes any calculation that treats them as fixed single or double bonds inherently approximate.
Bond enthalpy reflects both electron density and the nature of orbital overlap.
A double bond is not twice as strong as a single bond, and a triple bond is not three times as strong, because:
Additional pi bonds contribute less stabilisation per bond than sigma bonds
Electron repulsion increases with more shared electrons
Bond length shortens, affecting stability and energy requirements
Thus, the increase is significant but not proportional.
If the reaction involves unusual bonding environments, hydrogen bonding, or changes in physical state, the true enthalpy change may differ from estimates.
Average bond enthalpies apply only to gaseous species, so reactions involving liquids or solids introduce additional energetic factors that are not accounted for.
These limitations can cause a predicted exothermic reaction to appear endothermic, or vice versa, when more accurate thermochemical data are used.
Practice Questions
Define the term average bond enthalpy and explain why values from data tables may differ from experimentally determined enthalpy changes.
(2 marks)
1 mark: States that average bond enthalpy is the energy required to break one mole of a given type of bond in the gaseous state, averaged across many compounds.
1 mark: Explains that values differ because bond enthalpies are averages and do not reflect specific molecular environments present in real substances.
Propane (C3H8) combusts in oxygen to form carbon dioxide and water.
Using the average bond enthalpies below, estimate the enthalpy change for the complete combustion of propane.
C–H: 413 kJ mol⁻¹
C–C: 348 kJ mol⁻¹
O=O: 498 kJ mol⁻¹
C=O (in CO2): 805 kJ mol⁻¹
O–H: 463 kJ mol⁻¹
(a) State all the bonds broken in the reactants.
(b) State all the bonds formed in the products.
(c) Use the values above to estimate the enthalpy change for the reaction.
(d) Explain why the value obtained may differ from one calculated using standard enthalpies of formation.
(5 marks)
(a) Bonds broken (1 mark)
8 C–H bonds
2 C–C bonds
5 O=O bonds
(b) Bonds formed (1 mark)
6 C=O bonds (in 3 CO2 molecules)
8 O–H bonds (in 4 H2O molecules)
(c) Correct structure of calculation showing: total energy for bonds broken minus total energy for bonds formed (2 marks)
Award marks for:
Identifying correct totals for energy in and energy out
Subtracting correctly to obtain a large negative value (exact numerical answer not required for full marks if method is correct)
(d) Reason for discrepancy (1 mark)
Average bond enthalpies are mean values for bonds in the gaseous state, whereas standard enthalpies of formation use actual thermodynamic data for substances in their standard states.

