OCR Specification focus:
‘Define standard conditions, standard states and enthalpy changes of reaction, formation, combustion and neutralisation, using precise chemical language.’
Understanding standard conditions, standard states, and key definitions of enthalpy changes is essential for accurate thermochemical descriptions and comparisons in OCR A-Level Chemistry, supporting consistent and reliable energetic analysis.
Standard Conditions in Thermochemistry
Standard conditions provide a common reference point for comparing enthalpy changes in chemical reactions. They ensure that values reported across different experiments can be interpreted consistently.
Definition of Standard Conditions
Standard conditions refer to a set of precisely defined physical requirements used when reporting enthalpy changes.
Standard Conditions: The specific set of conditions including pressure of 100 kPa, temperature of 298 K (25 °C) unless otherwise stated, and solutions of 1.0 mol dm⁻³ concentration.
These conditions must not be confused with standard temperature and pressure (STP), which differ from the IUPAC-aligned standard conditions used in thermochemistry. The purpose of adopting standard conditions is to allow meaningful comparison between different experimentally determined enthalpy values.
Standard States
A substance’s standard state is fundamental to defining all standard enthalpy changes used throughout physical chemistry.
Definition of Standard State
The standard state indicates the most stable physical form of a substance under standard conditions.
Standard State: The pure and most stable physical form of an element or compound under 100 kPa and at a specified temperature, usually 298 K.
Examples include oxygen as O₂(g), water as H₂O(l), and carbon as C(s, graphite). These conventions ensure clarity when constructing enthalpy cycles and interpreting thermochemical data.
For example, oxygen has the standard state O₂(g), while carbon’s standard state is C(graphite), not diamond.

Graphite powder, cut diamond crystals and a pencil tip showing two allotropes of carbon. Graphite is more thermodynamically stable at standard conditions and is therefore chosen as the standard state of carbon. This visual emphasises that standard states are defined by stability rather than appearance. Source
Enthalpy Changes: Key Definitions
OCR requires precise use of thermochemical language when defining enthalpy changes, as these definitions underpin Hess’ law, bond enthalpy calculations, and calorimetric analysis.
Enthalpy Change of Reaction (ΔHᵣ)
This describes the enthalpy change associated with a reaction according to its balanced chemical equation.
Enthalpy Change of Reaction (ΔHᵣ): The enthalpy change when a reaction occurs in the molar quantities shown in the chemical equation under standard conditions, with all reactants and products in their standard states.
This definition applies to any reaction, whether exothermic or endothermic, and is the basis for other specific enthalpy definitions.
Enthalpy Change of Formation (ΔHf°)
Formation enthalpy data allow indirect calculation of otherwise inaccessible reaction enthalpies using Hess' law.
Standard Enthalpy Change of Formation (ΔHf°): The enthalpy change when 1 mole of a compound is formed from its elements in their standard states under standard conditions.
A typical formation reaction follows the structure “elements → compound”, with stoichiometry adjusted so that exactly one mole of product is created.
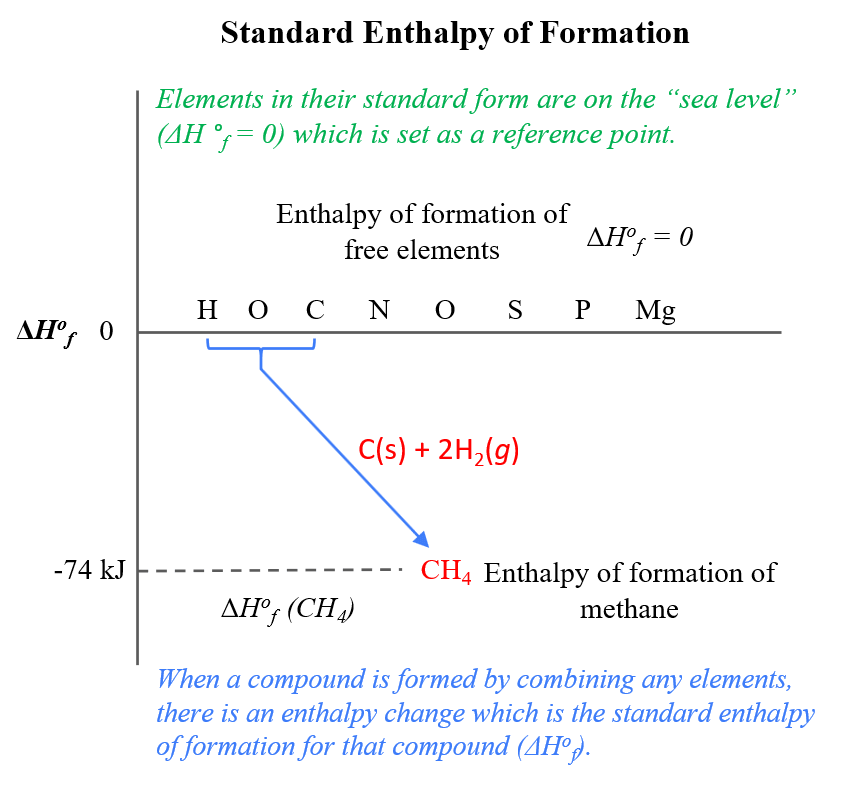
Standard enthalpy of formation diagram for methane. Elements in their standard states appear at ΔHf = 0, with CH₄ shown at a lower enthalpy to indicate an exothermic formation. This reinforces that formation enthalpies are measured relative to elements in their standard states under standard conditions. Source
Enthalpy Change of Combustion (ΔHc°)
Combustion enthalpies are widely used due to the complete and highly exothermic oxidation of fuels.
Standard Enthalpy Change of Combustion (ΔHc°): The enthalpy change when 1 mole of a substance is completely combusted in oxygen under standard conditions, with all reactants and products in their standard states.
Combustion values are typically negative because these reactions release large amounts of energy as heat.
Enthalpy Change of Neutralisation (ΔHₙₑᵤ°)
Neutralisation is central to acid–base chemistry and calorimetry, especially in aqueous systems.
Standard Enthalpy Change of Neutralisation (ΔHₙₑᵤ°): The enthalpy change when 1 mole of water is formed by reaction between an acid and a base under standard conditions, with all reactants and products in their standard states.
In aqueous solutions, neutralisation typically involves the reaction of H⁺(aq) and OH⁻(aq) to produce H₂O(l). For strong acids and strong bases, values tend to be similar because the ionic process is effectively the same.
Importance of Standardisation in Enthalpy Measurements
Precise definitions ensure that enthalpy values can be compared, tabulated, and applied correctly when constructing enthalpy cycles or applying Hess’ law. Without standardisation, enthalpy data from calorimetry, combustion analysis, or databases would be incompatible.
Why Standard Conditions and States Matter
Using standard conditions and states allows chemists to:
Compare enthalpy values from different reactions reliably.
Construct accurate enthalpy cycles for indirect calculations.
Apply Hess’ law consistently across varied reaction systems.
Use tabulated ΔHf°, ΔHc°, and ΔHₙₑᵤ° values confidently in calculations.
Standardisation therefore ensures uniformity and clarity in thermochemical work across OCR A-Level Chemistry and wider scientific practice.
Key Features to Recognise in Thermochemical Definitions
When learning enthalpy terminology, students should note:
All definitions refer to 1 mole of a specified substance or product.
Standard conditions always include 100 kPa and usually 298 K unless stated otherwise.
Reactants and products must be in their standard states.
Formation uses elements, combustion uses oxygen, and neutralisation uses acid–base reactions.
These definitions support further subtopics such as calorimetric methods, average bond enthalpies, and Hess’ law.
A strong command of this terminology allows accurate interpretation of enthalpy diagrams, energy profiles, and data tables essential for further physical chemistry topics.
FAQ
Standard conditions specify the physical environment (100 kPa, 298 K, and 1.0 mol dm⁻³ for solutions).
Standard state refers to the most stable physical form of a substance under those conditions.
Both are needed because enthalpy changes must be measured relative to a clearly defined form of each substance, under fixed and reproducible conditions.
298 K is closer to normal laboratory temperatures, meaning enthalpy values measured experimentally require fewer temperature corrections.
It also aligns with IUPAC conventions and ensures consistency across thermochemical data tables.
Yes. A standard state is temperature-dependent because the most stable phase can vary with temperature.
For example:
At 298 K, water’s standard state is liquid.
At higher temperatures, its most stable state may become gaseous, changing its standard state accordingly.
Assigning zero to elements in their standard states creates a reference baseline.
This convention allows chemists to:
Compare enthalpy changes across different substances
Construct enthalpy cycles easily
Apply Hess’ law without needing absolute enthalpy values
No. Only the most stable allotrope at standard conditions is assigned a value of zero.
Other allotropes have positive standard enthalpy of formation values because energy would be required to convert the stable form into them.
Practice Questions
Define the term standard enthalpy change of formation and state the standard conditions used when reporting enthalpy changes.
(2 marks)
Correct definition: Enthalpy change when 1 mole of a compound is formed from its elements in their standard states under standard conditions. (1 mark)
States correct standard conditions: 100 kPa pressure, 298 K temperature, solutions at 1.0 mol dm⁻³ concentration. (1 mark)
A student states:
"Diamond should be used instead of graphite when defining standard enthalpy changes because diamond is more valuable."
Using your knowledge of standard states and standard enthalpy definitions, explain why this statement is incorrect.
In your answer, refer to:
what a standard state is
why graphite is used for carbon
the importance of consistency in enthalpy calculations.
(5 marks)
Identifies that a standard state is the most stable physical form of an element under standard conditions. (1 mark)
States that graphite is the most stable allotrope of carbon under standard conditions. (1 mark)
Explains that diamond is not used because it is not the standard state (less thermodynamically stable). (1 mark)
States that enthalpy definitions rely on standard states to ensure consistent reference points. (1 mark)
Explains that using diamond instead of graphite would produce inconsistent or incorrect enthalpy values. (1 mark)

