OCR Specification focus:
‘Apply IUPAC rules to name organic compounds within the specification’s functional groups, including first ten alkanes and corresponding alkyl groups; use systematic names to avoid ambiguity.’
Systematic naming in organic chemistry ensures every compound has a unique, unambiguous name. IUPAC nomenclature provides structured rules that allow chemists to identify, describe, and communicate organic molecules precisely.
IUPAC Nomenclature Essentials
IUPAC nomenclature gives a universal method for naming organic compounds so structures can be interpreted unambiguously. Names are constructed from components that describe the carbon chain, functional groups, and substituents. Students should understand how these features combine to form systematic names and why consistency is essential in organic chemistry.
Core Principles of Systematic Naming
IUPAC naming relies on selecting and describing the longest continuous carbon chain, identifying all functional groups, and assigning locants (numbers) to indicate their positions. The objective is to eliminate ambiguity, especially in molecules with similar structures.
The first ten alkanes form the basis of many names because their stem names (meth–, eth–, prop–, but–, pent–, hex–, hept–, oct–, non–, dec–) are used widely across functional groups and homologous series. Corresponding alkyl groups follow the same pattern, replacing the final –ane with –yl, such as methyl or ethyl.
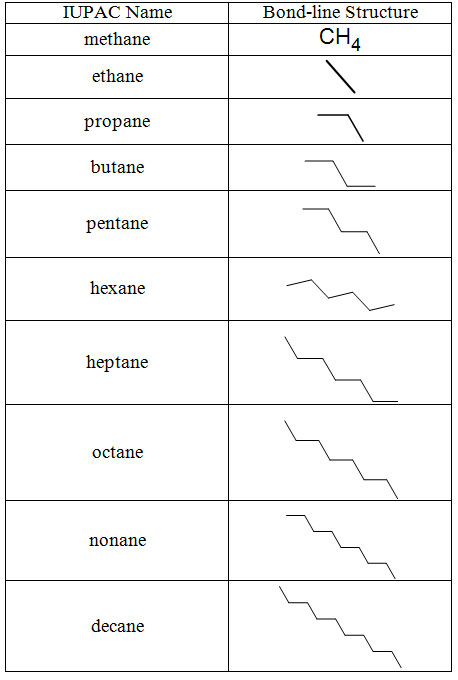
Table of the first ten straight-chain alkanes, showing each IUPAC name, molecular formula and bond-line structure. This visual reinforces the link between the meth–, eth–, prop– … dec– prefixes and the number of carbon atoms in the parent chain. It directly supports OCR’s emphasis on secure recall of the first ten alkanes. Source
Defining Key Organic Terms
Understanding several foundational terms is essential before applying nomenclature rules.
Functional group: A specific arrangement of atoms that determines a molecule’s chemical properties and reactivity.
A single sentence must appear here before introducing the next definition block.
Alkyl group (R): A substituent formed by removing one hydrogen from an alkane, usually represented as a generic carbon chain fragment.
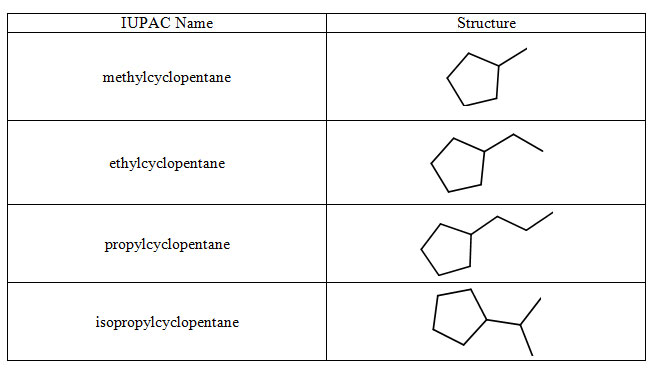
Table of common alkyl substituents derived from alkanes, each drawn as a short fragment attached to a cyclopentane ring. The figure reinforces how methyl, ethyl and larger groups are treated as side chains in IUPAC names. The use of a cyclopentane parent ring goes slightly beyond the OCR requirement but only serves as a neutral scaffold for displaying the substituents. Source
These terms underpin how substituents and parent chains are chosen when constructing names.
Parent Chain Selection
Choosing the parent chain is a critical first step in naming. The parent chain:
Must be the longest continuous chain of carbon atoms.
Must include the principal functional group if present.
Determines the stem and suffix of the name.
If multiple chains of equal length exist, the chain with the greatest number of substituents or the highest-priority functional group is selected.
Substituents and Their Placement
Once the parent chain is identified, all substituents must be located and named. Substituents are labelled using numbers (locants) to show their positions along the chain.
When numbering the carbon chain, choose the direction that:
Gives the lowest possible locant to the principal functional group.
Minimises the locants of substituents if no functional group has priority.
Common substituents include:
Alkyl groups (methyl, ethyl, propyl)
Halogens (fluoro, chloro, bromo, iodo)
Multiple identical substituents use prefixes di-, tri-, tetra-. Locants are separated by commas, and numbers are separated from letters by hyphens.
Functional Group Suffixes and Priorities
Suffixes indicate the main functional group. Some examples include:
–ane for alkanes
–ene for alkenes
–ol for alcohols
–al for aldehydes
–one for ketones
Functional groups also have a priority order for determining numbering direction. Higher-priority groups such as carboxylic acids, aldehydes, and alcohols influence both the suffix and the numbering of the carbon chain.
Using Alkyl Groups in Naming
The specification highlights the importance of the first ten alkyl groups. These groups appear frequently as substituents, and students should recognise their names and structures. Alkyl groups follow the pattern:
methane → methyl
ethane → ethyl
propane → propyl
butane → butyl
Longer chains follow the same systematic stem formation.
Constructing Systematic Names
Names are assembled by combining three key components:
Prefix – substituents in alphabetical order.
Stem – based on the parent chain length.
Suffix – main functional group.
Important guidelines include:
Alphabetical order ignores prefixes like di-, tri-, and tetra-.
Functional group suffixes replace the final –e of alkane stems when necessary.
The placement of numbers must be clear, consistent, and unambiguous.
Eliminating Ambiguity
A central purpose of IUPAC nomenclature is preventing ambiguity. Systematic names avoid confusion by:
Providing explicit locants for every substituent.
Using a single, standardised naming system.
Following consistent rules across homologous series.
Ambiguous or outdated common names such as isobutane or acetone should be avoided unless used alongside systematic names in context.
Representing Branched Structures Clearly
Branched alkanes often require careful application of naming rules. Key considerations include:
Identifying complex substituents, which may themselves require numbering.
Using parentheses to enclose substituent names when needed.
Ensuring alphabetical ordering compares the first meaningful letter of each substituent.
Illustration of short-line (skeletal) structures for simple alkanes such as propane. It shows how each bend or end of a line represents a carbon atom, with implied hydrogens completing four bonds. The figure slightly anticipates later use of skeletal formulae across the course but remains fully aligned with the representational skills expected by OCR. Source
Avoiding Common Errors
Students often make predictable mistakes when naming organic molecules. Frequent issues include:
Misidentifying the longest carbon chain.
Incorrect numbering direction.
Ignoring functional group priority.
Omitting locants for double bonds or substituents.
Understanding these pitfalls strengthens application of the rules.
Systematic Naming Across Functional Groups
Although this subsubtopic focuses on alkanes and alkyl groups, the same naming principles apply across aldehydes, ketones, carboxylic acids, halogenoalkanes, and alkenes within the OCR specification. Recognising functional-group suffixes and applying consistent numbering enhances confidence when interpreting unfamiliar structures.
Final Notes on Prefix–Stem–Suffix Integration
A well-constructed systematic name provides a complete map of a molecule’s structure. By combining prefixes (substituents), stems (chain length), and suffixes (functional groups), IUPAC nomenclature offers clarity and precision essential for success in A-Level Chemistry.
FAQ
Alphabetical order is based on the first meaningful letter of each substituent name. Prefixes such as di, tri and tetra are ignored when alphabetising.
Alphabetical priority is determined using:
The first letter of the substituent (e.g., ethyl before methyl).
Complex substituents being alphabetised according to their root name, not the first letter inside brackets.
When numbering could give equal locants, the deciding factor is the position of the substituent that appears first alphabetically.
If groups are identical, the chain is numbered to give the lowest possible set of locants overall, not just the first one.
Complex substituents are named like smaller organic molecules and placed in parentheses within the full IUPAC name.
Key rules include:
Numbering the substituent from the carbon attached to the main chain.
Using prefixes within the parentheses if the substituent has more than one branch.
Alphabetising using the first letter of the substituent name outside the parentheses.
The parent chain must be the longest continuous chain of carbon atoms, even if it appears to “bend” or change direction.
If multiple chains share the same length, select the one containing:
The highest-priority functional group.
The greatest number of substituents.
The arrangement that gives the lowest overall locants.
A locant is only needed when a functional group can appear in multiple possible positions on the carbon skeleton.
No locant is required when:
The functional group is fixed at carbon 1 (e.g., carboxylic acids).
Only one arrangement is structurally possible for that functional group.
Practice Questions
Name the following organic compound using full IUPAC rules:
CH3–CH2–CH(CH3)–CH2–Br
(2 marks)
Correct IUPAC name: 2-methylbutan-1-yl bromide OR 1-bromo-2-methylbutane
Mark breakdown:
1 mark for correct identification of parent chain (butane).
1 mark for correct substituent and numbering (2-methyl and bromo at carbon 1).
A compound has the molecular formula C5H12O.
Using IUPAC rules, answer the following:
a) State what is meant by the term functional group.
b) Draw and name two different structural isomers containing an alcohol functional group.
c) Explain why these isomers have different IUPAC names even though they share the same molecular formula.
(5 marks)
a) Definition of functional group (1 mark):
Functional group is the specific group of atoms responsible for the characteristic reactions of a compound.
b) Two structural isomers with alcohol functional groups (2 marks):
Any two correct structures and names from the following:
Pentan-1-ol
Pentan-2-ol
2-methylbutan-1-ol
3-methylbutan-1-ol
Award 1 mark per correct structure–name pair (max 2 marks).
c) Explanation of name differences (2 marks):
1 mark: Isomers differ in the arrangement of atoms / carbon skeleton / position of the OH group.
1 mark: Different structural arrangements require different systematic names to avoid ambiguity.

