OCR Specification focus:
‘Define homologous series; use general formulae to predict members; understand recurring functional groups and CH₂ increments across a series for property and reactivity trends.’
Homologous series underpin the study of organic chemistry by showing repeating structural patterns, predictable formulae, and gradual changes in characteristics across related compounds sharing the same functional group.
Homologous Series: Core Principles
A homologous series forms the basis for organising and predicting behaviour in organic chemistry because members share essential structural features.
Homologous series: A family of organic compounds with the same functional group and general formula, in which each successive member differs by a CH₂ unit.
Members of a homologous series exhibit consistent chemical reactivity due to their shared functional group, while physical properties change gradually because of increasing chain length. This pattern allows chemists to predict the formulae and likely characteristics of unfamiliar members.
Functional Groups in Homologous Series
A functional group is the distinctive atom or group of atoms responsible for the chemical behaviour of a series.
Functional group: The atom or group of atoms in an organic molecule responsible for its characteristic reactions.
Functional groups are constant within a homologous series, linking compounds through shared reactivity despite differences in chain length. Students must recognise how recurring functionality ensures similar reaction pathways across a series.
A homologous series also shares a general formula, which expresses the algebraic relationship between carbon and hydrogen atoms (and sometimes heteroatoms) for all its members.
General formula: An algebraic expression representing the composition of all members of the same homologous series.
General formulae enable rapid deduction of possible compounds before structural or displayed formulae are drawn.
CH₂ Increments and Their Consequences
Each member of a homologous series differs from the next by a CH₂ increment, creating systematic structural progression. This small increase in chain length influences intermolecular forces and physical properties.
Each successive member of a homologous series differs from the previous one by a single –CH₂– unit in the carbon chain.
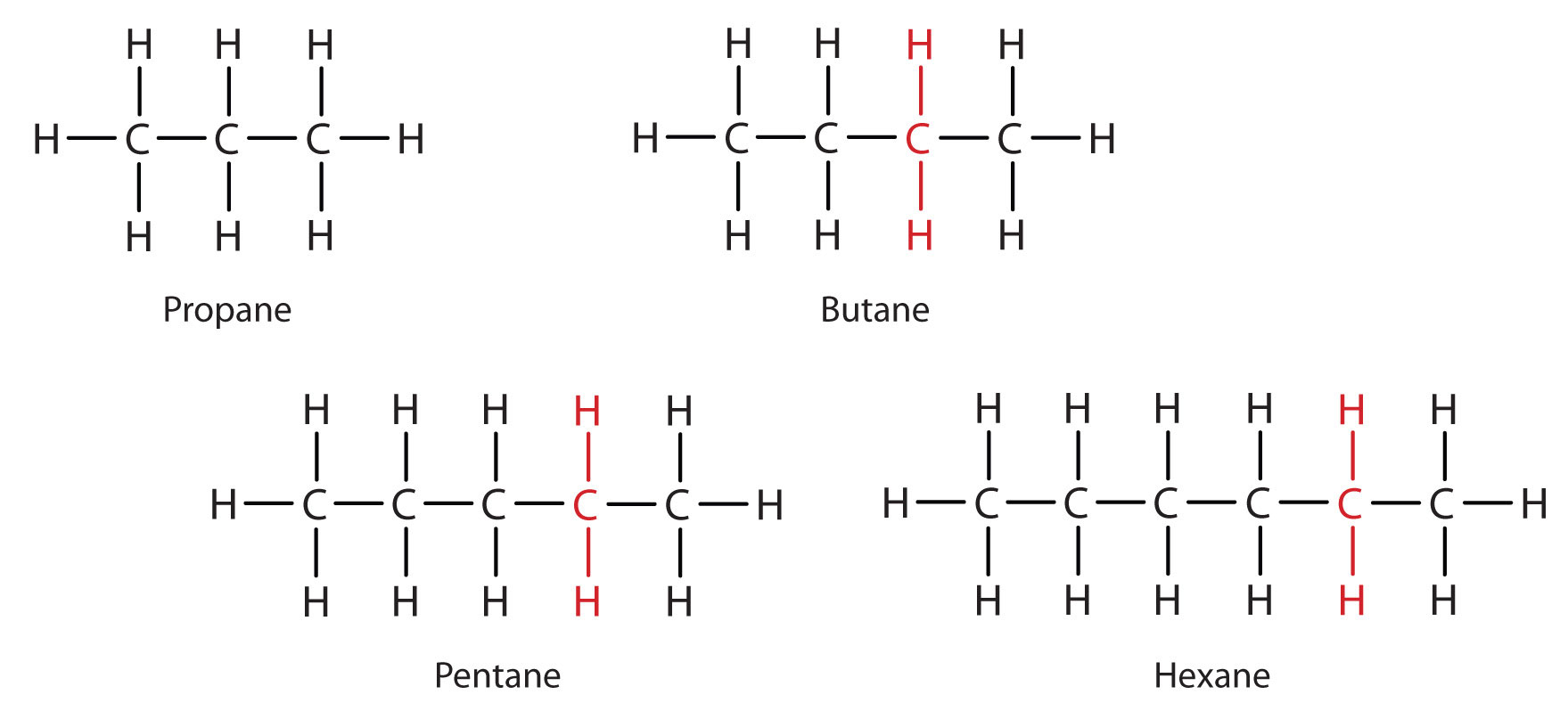
Members of a homologous series of alkanes from propane to hexane, each increasing by one –CH₂– unit, illustrating the predictable structural pattern across the series. Source
Effects of CH₂ Increments on Physical Properties
Because intermolecular forces rise with chain length, homologous series show characteristic trends:
Increased boiling points as molecules become larger and exhibit stronger induced dipole–dipole interactions.
Increased melting points, although the trend may not be perfectly smooth due to packing efficiency.
Increased viscosity in longer-chain members.
Decreased volatility with increasing molecular size.
Although these changes differ between series, the consistent CH₂ difference makes the patterns predictable.
A single sentence separates this from the next definition. Members also show predictable chemical features due to their shared functional group.
Alkyl group (R): A substituent formed by removing one hydrogen atom from an alkane, typically represented as R–.
The CH₂ progression also explains why homologous series can be extended indefinitely, with each new compound building upon the same structural foundation.
General Formulae and Predicting Members
General formulae allow chemists to anticipate the composition and likely structure of any compound in a series before examining its full representation. This predictive power is a key skill emphasised by the OCR specification.
Using General Formulae
To apply general formulae effectively, students must recognise patterns across common series:
Alkanes: CₙH₂ₙ₊₂
Alkenes: CₙH₂ₙ
Alcohols: CₙH₂ₙ₊₂O
Carboxylic acids: CₙH₂ₙO₂
These formulae demonstrate how compound families are built systematically, enabling prediction of molecular formulae from integer values of n.
General formulae also help check whether a proposed molecular formula belongs to a given series or whether additional structural information is needed.
A general formula captures the relationship between carbon and hydrogen atoms across an entire homologous series and allows you to predict the formula of any member.
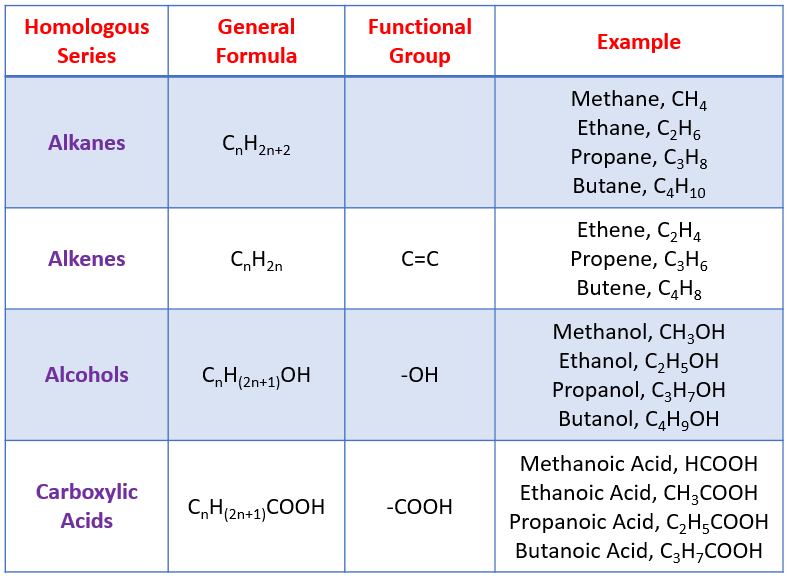
A summary table of several homologous series showing their shared functional groups, general formulae, and representative molecules, illustrating how predictable structural patterns extend across different organic families. Source
Relationship Between Structure and Reactivity
Because compounds share the same functional group and differ only by chain length, their chemical reactions are usually very similar. Trends arise mainly from physical rather than chemical differences:
Reactivity patterns remain consistent across the series.
Reaction mechanisms are typically identical.
Reaction conditions (e.g., catalysts, temperatures) vary only slightly, if at all.
The OCR specification highlights how understanding these trends enables confident prediction of reactivity across all members.
A single bridging sentence before the next definition clarifies another structural term relevant to homologous series. Structural language in organic chemistry provides essential tools for describing variation within and across series.
Aliphatic: Describes organic compounds consisting of straight-chain, branched or non-aromatic rings of carbon atoms.
Understanding aliphatic structures supports recognition of homologous patterns in many series taught at A-Level.
Structural Implications of Homology
The repeating CH₂ framework in a homologous series ensures predictable transformations when applying structural, displayed, or skeletal formulae. This helps students translate between different organic representations accurately.
Formula Representations and Homology
Students must interpret and construct various formula types to express homologous relationships:
General formulae show algebraic patterns.
Structural formulae reveal connectivity.
Displayed formulae show all bonds explicitly.
Skeletal formulae present minimalist carbon frameworks.
Homology becomes especially clear in skeletal formulae, where chain extension by one vertex represents addition of a CH₂ unit.
Between structural representations and naming conventions, organic chemists rely on the predictable nature of homologous series for classification and communication.
Why Homologous Series Matter
The predictability inherent in homologous series allows organic chemistry to be studied systematically. Students can infer properties, behaviours, and compositions using only a functional group and a general formula, aligning precisely with OCR expectations for this subsubtopic.
FAQ
Solubility trends arise because increasing chain length strengthens London forces, reducing the influence of polar functional groups.
As a result, members become progressively less soluble in polar solvents such as water but remain miscible in non-polar solvents.
In homologous series containing oxygen-based functional groups, the polar region remains constant, but the growing non-polar hydrocarbon chain dominates solubility behaviour.
A molecular formula alone does not determine a functional group. Compounds sharing CnHx may contain different functional groups or arrangements that place them in distinct series.
For example, isomeric C4H8 may represent an alkene or a cycloalkane, which belong to different homologous families because their functional groups differ.
Chemists can anticipate reactivity pathways when designing syntheses because functional groups behave consistently across a series.
This allows predictable selection of reagents, reaction conditions and purification techniques.
It also means that once a synthetic route is established for one member, it can often be adapted for higher or lower homologues with minimal modification.
Although chain length strongly influences boiling points, molecular shape affects packing efficiency.
Branching reduces surface contact between molecules, weakening London forces and lowering boiling points.
Slight irregularities may also arise from subtle differences in intermolecular interactions or conformational stability between adjacent members.
General formulae provide a quick check to ensure the number of carbons, hydrogens and heteroatoms matches expectations for a series.
This allows students to spot structural errors such as an incorrect functional group, missing hydrogen, or miscounted carbon chain.
They also help verify whether a structure could logically belong to a given homologous family before detailed structural analysis takes place.
Practice Questions
Define the term homologous series and state one characteristic feature shared by all members.
(1–3 marks)
Homologous series defined as a family of organic compounds with the same functional group and general formula, each successive member differing by CH2. (1 mark)
Any one correct characteristic feature, such as similar chemical reactions or gradual changes in physical properties. (1 mark)
The homologous series of alkenes follows the general formula CnH2n.
a) Write the molecular formula for the next two members after propene.
b) Explain why alkenes show gradual changes in physical properties across the series but similar chemical reactivity.
(4–6 marks)
a)
Butene: C4H8. (1 mark)
Pentene: C5H10. (1 mark)
b)
Physical properties change gradually due to increasing chain length and stronger intermolecular forces as CH2 units are added. (1–2 marks)
Chemical reactivity remains similar because all alkenes contain the same functional group, the C=C double bond. (1 mark)
Explanation that the type of reactions depends on the functional group rather than chain length. (1 mark)
Total: 4–6 marks

