OCR Specification focus:
‘Use and interpret terms: functional group, alkyl group (R), aliphatic, alicyclic, aromatic; distinguish saturated vs unsaturated in terms of multiple carbon–carbon bonds and aromatic rings.’
Organic chemistry relies on a precise vocabulary that classifies molecules by structure and bonding; understanding these foundational terms enables clear communication and accurate prediction of chemical behaviour.
Functional Groups and Structural Terminology in Organic Chemistry
The Role of Functional Groups
A functional group is the characteristic atom or group of atoms in an organic molecule that determines its chemical properties and typical reactions. These groups define how molecules behave under different conditions and form the basis of organic classification.
Functional group: A characteristic group of atoms responsible for the chemical reactivity and properties of an organic compound.
Functional groups influence reactivity patterns, physical properties, and the naming of compounds. Their presence creates recurring behaviour across a homologous series, allowing trends in reactivity to be predicted more reliably.
Alkyl Groups: The ‘R’ Symbol
An alkyl group derives from an alkane by removing one hydrogen atom, and it is commonly represented by the symbol R. This allows generalised structures to be written efficiently, especially when the rest of the molecule is the focus.
Alkyl group (R): A fragment of an alkane formed by removing one hydrogen atom, used to represent a generic hydrocarbon chain in structures.
Using R keeps structures general, focusing attention on the functional group rather than the exact carbon chain attached.
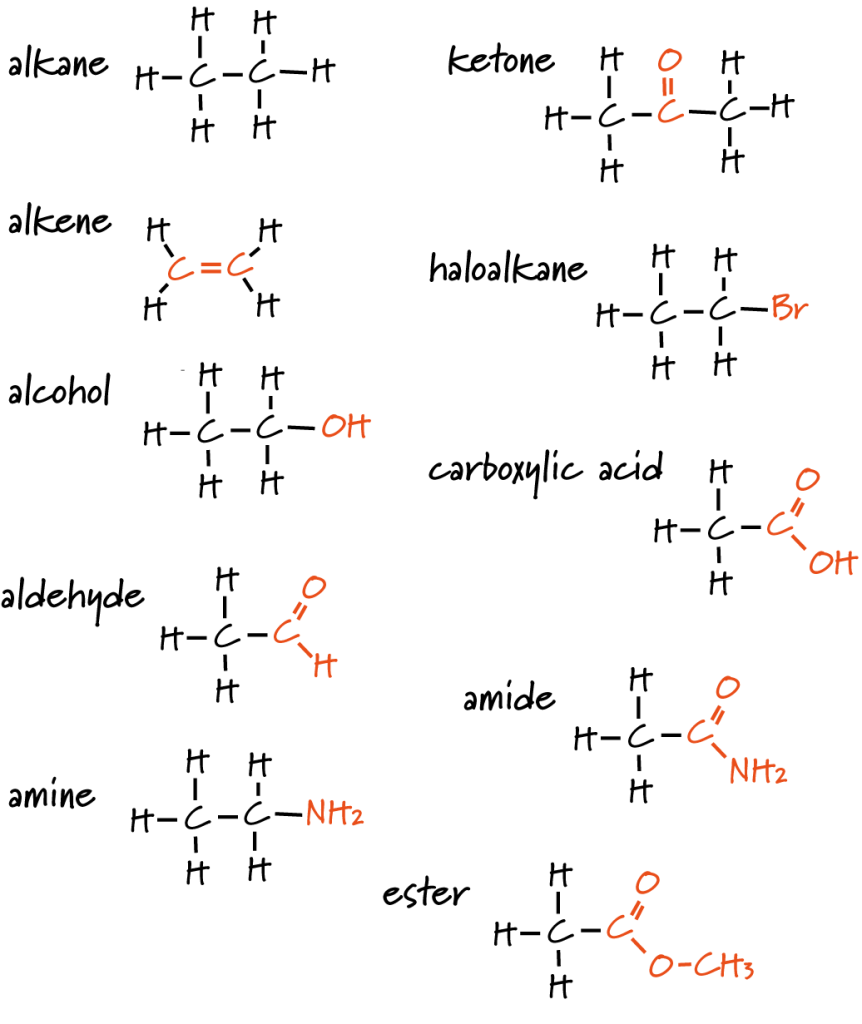
This image displays a range of organic molecules, each with its functional group emphasised in orange. It illustrates how different functional groups define chemical families and reactivity patterns. Some functional groups extend beyond syllabus requirements but reinforce the core concept. Source
Alkyl groups can vary in length and branching, but they behave similarly due to being composed solely of carbon and hydrogen. They often appear attached to functional groups in structural diagrams or general formulae.
Understanding Aliphatic Compounds
Organic molecules fall into several broad classifications, beginning with aliphatic compounds, which include straight-chain, branched, or non-aromatic ring structures. These compounds do not contain benzene rings.
Aliphatic: An organic compound containing carbon atoms joined in straight chains, branched chains, or non-aromatic rings.
Aliphatic molecules may be saturated or unsaturated, depending on the presence of double or triple carbon–carbon bonds. Students must take care not to confuse aliphatic with alicyclic or aromatic categories.
A normal sentence must appear here to ensure clear spacing between definition blocks before continuing the structural terminology.
Alicyclic Compounds and Ring Structures
Alicyclic compounds are a subset of aliphatic molecules arranged in ring structures but lacking the delocalised electron system associated with aromatic molecules. They share similarities with alkanes and alkenes but possess cyclic geometry.
Alicyclic: An aliphatic compound arranged in a ring structure without aromatic delocalisation.
Typical alicyclic representations include cyclohexane or cyclopentane, which are often drawn in simplified skeletal formulae. These compounds show typical aliphatic reactivity rather than aromatic stability.
Aromatic Compounds and Delocalised Rings
The term aromatic applies to compounds containing benzene rings or related ring systems with delocalised π-electrons. Aromatic compounds possess unique stability due to this electron delocalisation.
Aromatic: An organic compound containing a benzene ring or related delocalised ring system with characteristic stability.
Aromaticity gives rise to distinct naming conventions and predictable substitution reactions. In skeletal formulae, benzene is often shown as a hexagon with a circle to indicate delocalisation.
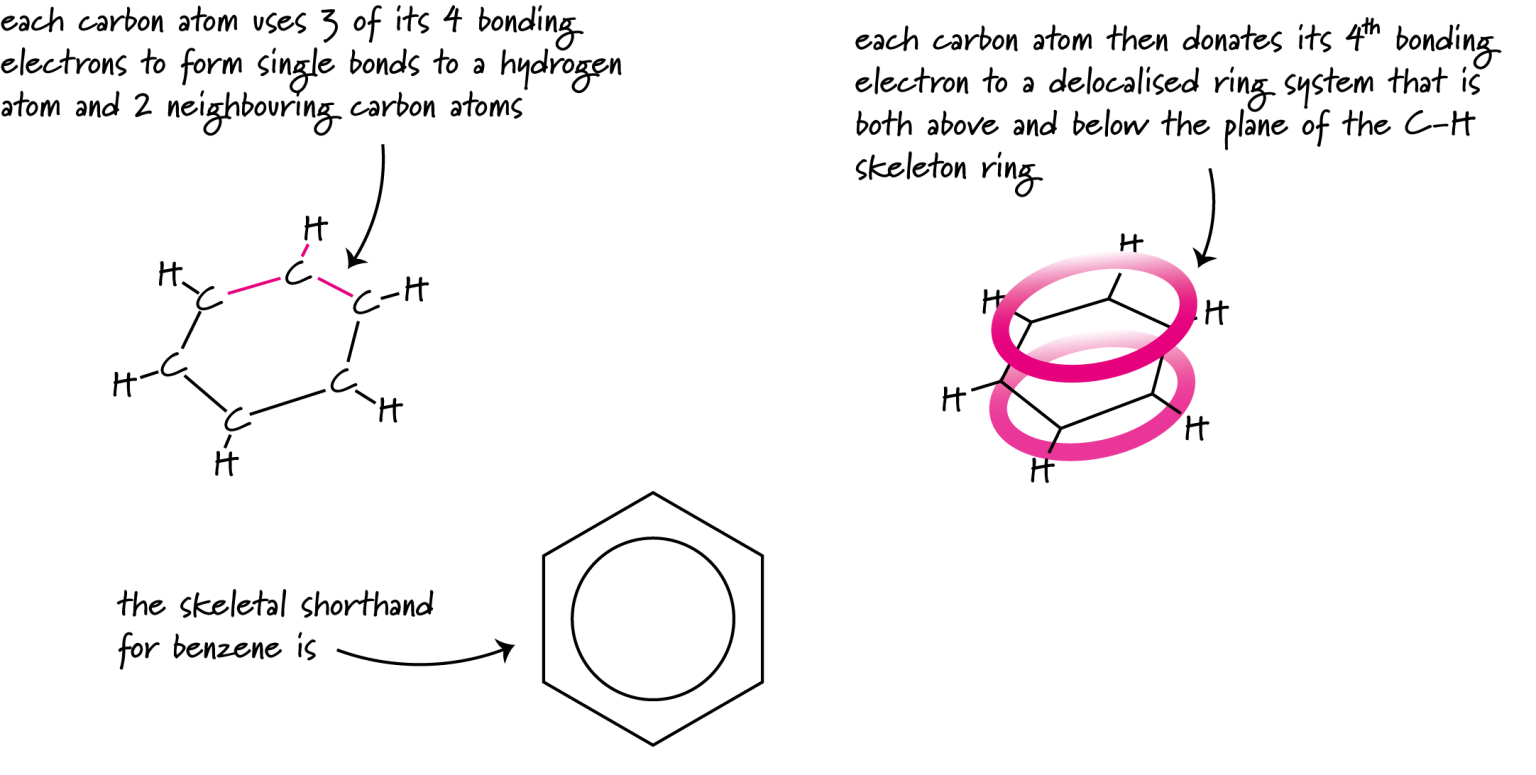
This figure shows benzene with its six-membered carbon ring and delocalised π-electron system. The shorthand hexagon-with-circle symbol reflects its aromatic stability. The depiction includes additional p-orbital detail beyond syllabus requirements to reinforce the idea of delocalisation. Source
Saturated and Unsaturated Structures
To classify organic molecules further, chemists distinguish between saturated and unsaturated structures. These terms refer specifically to the presence or absence of multiple carbon–carbon bonds.
Saturated: A molecule containing only single carbon–carbon bonds.
A sentence must appear here before the next definition to maintain proper formatting and clarity.
Unsaturated: A molecule containing one or more carbon–carbon double or triple bonds, or an aromatic ring.
The distinction is crucial when predicting reactivity. Unsaturated molecules, due to π-bonds or aromatic systems, typically undergo addition or substitution reactions depending on the nature of the multiple bond.
Using Structural Terms in Representations
Understanding terminology is essential when interpreting different formula types. Structural and skeletal formulae rely heavily on the terms introduced in this section.
Key applications include:
Identifying the functional group responsible for a molecule’s reactions.
Recognising the alkyl groups (R) present in displayed or skeletal formulae.
Distinguishing aliphatic, alicyclic, and aromatic frameworks.
Assessing whether a molecule is saturated or unsaturated based on bonding.
These classifications help students navigate complex structures and understand patterns in organic chemistry.
Relevance to the OCR A-Level Specification
The OCR specification emphasises accurate use of terminology to support structural interpretation and nomenclature. Students must confidently apply these terms when discussing molecular families, predicting properties, or constructing formulae.
Important specification-linked skills include:
Using functional-group vocabulary to classify compounds precisely.
Identifying alkyl substituents and applying the R notation effectively.
Distinguishing aliphatic, alicyclic, and aromatic structures when analysing organic molecules.
Linking saturation and unsaturation to the presence of multiple carbon–carbon bonds or aromatic rings.
Mastery of this terminology forms the foundation for subsequent topics, allowing students to approach mechanisms, reactivity trends, and nomenclature with clarity and accuracy.
FAQ
Look for the presence or absence of a benzene ring. A hexagon with a circle or alternating double bonds indicates an aromatic structure.
Alicyclic structures form closed rings but lack any delocalised system.
Aliphatic structures appear as straight or branched chains without ring formation.
Key indicators include:
Aromatic: ring with delocalisation symbol
Alicyclic: ring with no delocalisation
Aliphatic: no rings present
An alkyl group is always a hydrocarbon fragment with only C–H and C–C single bonds. It never contains heteroatoms such as O, N, or halogens.
A functional group contains at least one non-carbon atom or a multiple carbon–carbon bond.
Look for:
Presence of atoms other than C or H
Double or triple carbon–carbon bonds
Known characteristic groupings such as COOH or OH
Saturated structures contain only sigma bonds, making them generally less reactive toward electrophiles.
Unsaturated structures contain pi bonds or aromatic systems, which have higher electron density and attract electrophiles.
As a result:
Unsaturated compounds often undergo addition reactions
Aromatic compounds typically undergo substitution
Saturation affects stability, reactivity, and common reaction pathways
Not all aromatic compounds contain benzene. Some heterocyclic rings, such as pyridine or furan, are also aromatic.
To identify non-benzene aromaticity, look for:
A planar ring structure
Continuous p-orbital overlap around the entire ring
A stable delocalised electron system
These features ensure the compound meets the criteria for aromaticity even without benzene’s specific structure.
Alicyclic rings restrict rotation, increasing rigidity compared with open-chain structures. This can raise melting and boiling points.
Chemically, alicyclic compounds behave more like alkanes or alkenes, depending on whether their carbon–carbon bonds are single or multiple, but ring strain can make some reactions faster.
Important effects include:
Increased ring strain in small rings
Restricted conformations
Possible enhanced reactivity in strained systems
Practice Questions
Define the terms aliphatic and aromatic as used in organic chemistry.
(2 marks)
Aliphatic:
Molecule contains carbon atoms arranged in straight chains, branched chains, or non-aromatic rings. (1 mark)
Aromatic:
Molecule contains a benzene ring or other ring system with delocalised electrons. (1 mark)
Total: 2 marks
A compound has the molecular formula C6H12.
Using your knowledge of functional groups and structural terminology, answer the following:
a) State whether this molecular formula could represent a saturated or unsaturated compound, giving a reason.
b) Explain the difference between an alicyclic compound and an aromatic compound.
c) The compound is found to contain a carbon–carbon double bond. Identify the relevant structural term and describe what this tells you about its bonding.
(5 marks)
a)
The formula C6H12 may represent an unsaturated compound. (1 mark)
Because it fits the general formula for alkenes or could indicate a ring structure causing a reduction in hydrogen count. (1 mark)
b)
Alicyclic compounds are ring structures without aromatic delocalisation. (1 mark)
Aromatic compounds contain a delocalised electron system, typically in a benzene ring. (1 mark)
c)
Presence of a C=C bond identifies the compound as unsaturated / an alkene. (1 mark)
Indicates restricted rotation and the presence of a pi bond in addition to a sigma bond. (1 mark)
Total: 5 marks

