OCR Specification focus:
‘Account for boiling point changes with carbon-chain length and branching using induced dipole–dipole interactions (London forces); relate surface contact to intermolecular strength.’
Boiling points of alkanes depend on intermolecular forces rather than chemical reactivity. Understanding how chain length and branching influence London forces explains observable physical trends across the homologous series.
Boiling Point Trends in Alkanes
Alkanes are non-polar, saturated hydrocarbons, meaning they contain only C–C and C–H σ-bonds and lack permanent dipoles. As a result, their boiling points depend almost entirely on induced dipole–dipole interactions, also known as London forces, which operate between all molecules but dominate in alkanes. The strength of these forces is determined by how easily the electron cloud of a molecule can be distorted and by the degree of contact between neighbouring molecules.
London Forces and Electron Cloud Distortion
London forces arise from momentary fluctuations in electron distribution within a molecule, creating temporary dipoles that induce dipoles in neighbouring molecules. These very weak attractions accumulate across many atoms and become significant in larger hydrocarbons.
Induced dipole–dipole interactions (London forces): Temporary attractive forces caused by instantaneous dipoles that form when electron clouds fluctuate, inducing dipoles in adjacent molecules.
These intermolecular forces are the primary reason for boiling point variations across different alkanes. More extensive electron clouds distort more easily, creating stronger instantaneous dipoles.
Effect of Increasing Carbon-Chain Length
As carbon-chain length increases, the number of electrons in the molecule also increases. Larger molecules have larger electron clouds, which are more easily polarised, strengthening London forces. Stronger intermolecular forces require more energy to overcome, resulting in higher boiling points.
Key reasons why boiling points increase with chain length include:
More electrons → greater ease of electron cloud distortion.
Longer molecular surface area → more points of contact between molecules.
Stronger London forces overall, due to increased molecular size and mass.
These trends are consistent across the first ten alkanes and continue through the homologous series.
For a homologous series of straight-chain alkanes, boiling point increases smoothly as the number of carbon atoms in the chain increases.
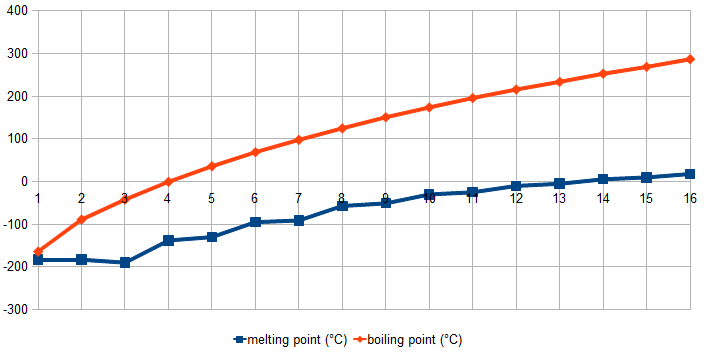
Graph illustrating the increase in boiling point with carbon number due to stronger London dispersion forces; melting points shown are additional context beyond OCR requirements. Source
Relationship Between Surface Contact and Intermolecular Strength
The extent of surface contact determines how effectively London forces can operate. Molecules with long, unbranched chains pack closely together, enabling stronger intermolecular attractions. Increased contact leads to greater cumulative intermolecular force strength.
Surface contact: The degree to which molecules can pack together and interact over a large area, influencing the strength of intermolecular forces.
Longer chains adopt flexible conformations that maximise surface interactions, reinforcing boiling point trends.
A normal sentence must follow before introducing another definition or equation, allowing continuity of explanation and contextual understanding.
Effect of Branching on Boiling Points
Branching significantly alters the physical behaviour of alkanes. Branched alkanes have lower boiling points than their straight-chain structural isomers. This arises from their more compact, spherical shapes, which reduce the overall surface area available for intermolecular contact.
Important reasons why branching decreases boiling point include:
More compact structure reduces surface area.
Fewer intermolecular contact points weaken cumulative London forces.
Lower effective polarisation due to reduced molecular elongation.
The position of branching also affects boiling point magnitude. A branch closer to the middle of the chain creates a more spherical molecule than one at the end, resulting in even lower boiling points.
More branched alkanes have lower boiling points than their straight-chain isomers because branching reduces the area of surface contact between molecules and so weakens London forces.
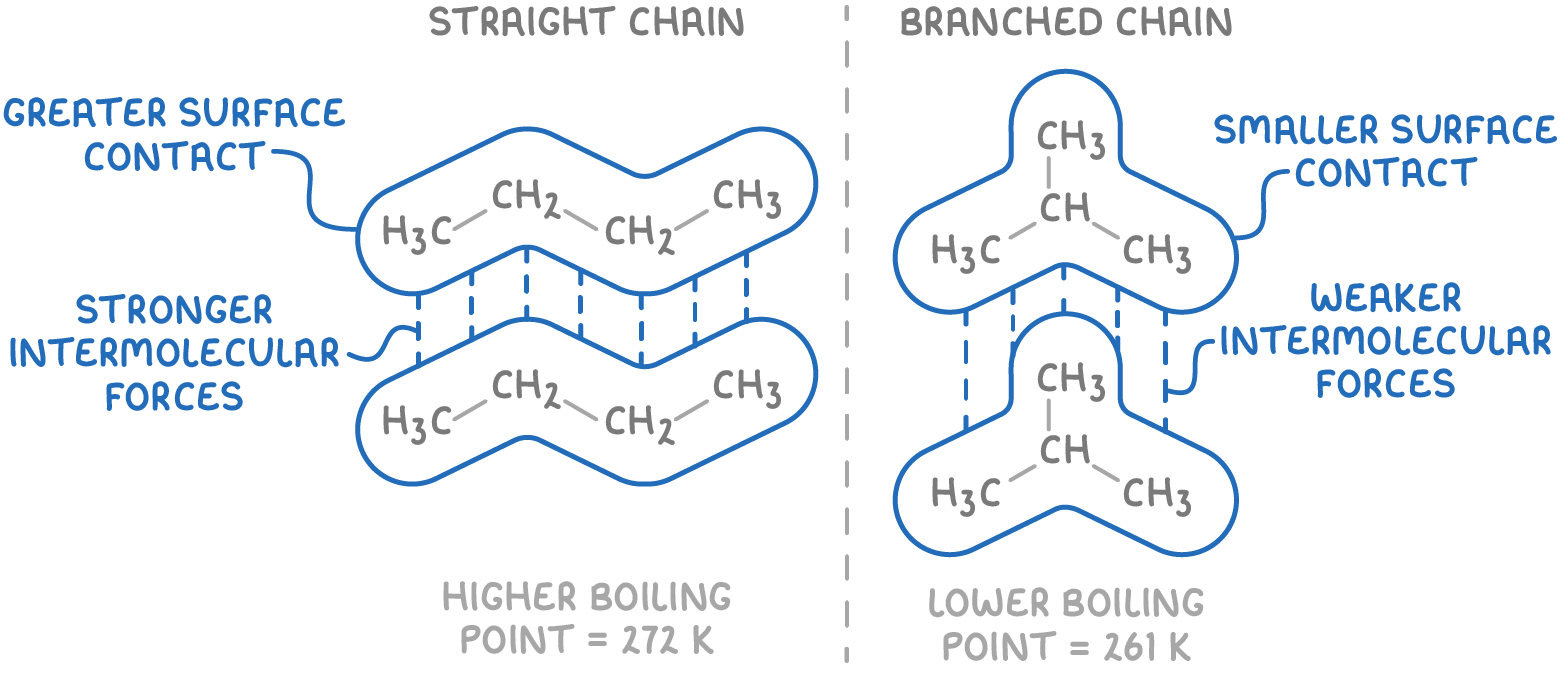
Diagram showing how straight-chain alkanes allow greater surface contact and stronger London forces compared with branched isomers, which pack less efficiently and have lower boiling points. Source
Explaining Trends Using Molecular Shape
Understanding the three-dimensional structure of alkanes is essential in explaining boiling point trends:
Straight-chain alkanes adopt elongated shapes, facilitating tight packing.
Increased branching produces shapes that do not align well with neighbouring molecules.
Reduced packing efficiency directly reduces London force strength.
These shape-driven variations illustrate how structural changes, even without altering functional groups or polarity, influence physical properties.
Comparison of Chain Length and Branching Effects
While both chain length and branching modify boiling points by influencing London forces, their effects act in opposite directions:
Increasing chain length strengthens London forces, raising boiling points.
Increasing branching weakens London forces, lowering boiling points.
Thus, an isomer with a longer unbranched chain will always have a higher boiling point than a more branched isomer with the same molecular formula. This relationship underscores the importance of molecular structure in determining physical properties of organic compounds.
Why Alkanes Follow Predictable Trends
Because alkanes lack permanent dipoles and other intermolecular forces (such as hydrogen bonding), their boiling point behaviour is particularly regular and predictable. The dominant London forces respond directly to electron number and molecular geometry, making alkanes an ideal model system for studying the effect of structure on boiling point.
Bullet-point recap of key structural influences:
Chain length: more electrons, larger polarisation, stronger London forces.
Branching: reduced surface contact, weaker London forces.
Geometry: shape determines molecular packing efficiency.
Linking Microscopic Interactions to Macroscopic Observations
The OCR specification emphasises relating surface contact to intermolecular strength, a crucial idea for connecting microscopic molecular behaviour with bulk physical properties observed in the laboratory. Stronger London forces manifest as higher boiling points because additional thermal energy is required to separate molecules during vaporisation.
In practical terms, these principles explain why hexane is liquid at room temperature, whereas methane is a gas: methane’s small electron cloud and minimal surface area produce extremely weak London forces. Conversely, larger hydrocarbons show progressively higher boiling points because their extensive electron clouds enhance intermolecular attraction.
Understanding these structural and intermolecular factors equips students to rationalise boiling point data across the homologous series and recognise how branching disrupts predictable upward trends.
FAQ
London forces operate in all molecules, but in alkanes they are particularly significant because there are no permanent dipoles or hydrogen bonds.
The magnitude of these forces depends largely on the number of electrons and the shape of the molecule.
Other non-polar molecules, such as noble gases or symmetrical covalent molecules, show similar trends, but alkanes often have stronger London forces due to larger electron clouds and extended chain structures.
Although molecular mass contributes to electron cloud size, boiling points are more strongly affected by how closely molecules can pack together.
A larger surface area creates more opportunities for instantaneous dipole interactions.
Two molecules with similar masses can have very different boiling points if one is elongated and the other compact, because the elongated one allows greater surface contact.
Cyclic alkanes generally have higher boiling points than their straight-chain isomers, despite having the same molecular formula.
This is because:
Rings create rigid, compact structures with multiple points of contact.
The enforced shape increases intermolecular interactions compared with comparable branched alkanes.
Their boiling points still increase with ring size, but the effect of molecular shape becomes more prominent.
Multiple branching lowers boiling point further because it increasingly reduces surface contact.
Key effects include:
Each additional branch increases molecular compactness.
Highly branched molecules resemble spheres, which pack poorly.
Fewer contact points weaken London forces significantly.
As a result, heavily branched hydrocarbons can have dramatically lower boiling points than their straight-chain equivalents.
Even when two branched alkanes appear similarly compact, small structural differences influence how molecules interact.
Factors that create variation include:
Branch position: central branches reduce surface contact more than terminal ones.
Branch length: longer substituents increase electron cloud size slightly.
Symmetry: more symmetrical molecules pack less effectively.
These subtle structural features lead to measurable differences in intermolecular forces and therefore boiling points.
Practice Questions
Explain why the boiling point of octane is higher than the boiling point of butane.
(2 marks)
Award 1 mark for each valid point:
Octane has a longer carbon chain with more electrons, leading to stronger induced dipole–dipole (London) forces.
More surface contact between octane molecules increases intermolecular attraction, requiring more energy to separate them.
Hexane and 2-methylpentane have the same molecular formula but different boiling points.
(a) State which compound has the higher boiling point.
(b) Explain, in terms of intermolecular forces and molecular structure, why their boiling points differ.
(5 marks)
(a)
Hexane has the higher boiling point. (1 mark)
(b)
Award up to 4 marks for the following:
Hexane is a straight-chain alkane, whereas 2-methylpentane is branched. (1 mark)
Straight-chain alkanes have a larger area of surface contact between molecules. (1 mark)
Greater surface contact results in stronger induced dipole–dipole (London) forces. (1 mark)
Stronger London forces mean more energy is required to separate the molecules, giving hexane the higher boiling point. (1 mark)

