OCR Specification focus:
‘Alkanes are saturated hydrocarbons with C–C and C–H σ-bonds formed by direct orbital overlap; σ-bonds allow free rotation around C–C bonds.’
Alkanes form a fundamental class of organic molecules whose behaviour is governed by the nature of the σ-bonds between carbon and hydrogen atoms. Understanding σ-bond formation and the resulting free rotation around C–C bonds provides an essential basis for explaining the structural features, conformational flexibility and relatively low reactivity of alkanes across the wider organic chemistry specification.
σ-Bond Formation in Alkanes
Alkanes are defined as saturated hydrocarbons, containing only single covalent bonds between carbon atoms. Each of these C–C and C–H bonds is a σ-bond, created by direct (end-to-end) overlap of orbitals.
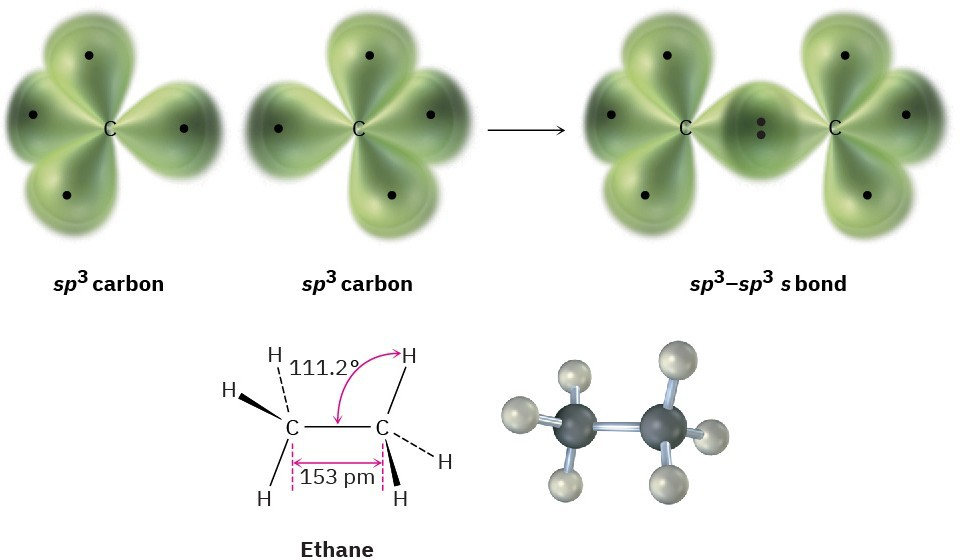
The diagram illustrates σ-bonding in ethane, with sp³–sp³ overlap forming the C–C σ-bond and sp³–1s overlaps forming C–H σ-bonds. Additional labelled angles and distances extend slightly beyond this subsubtopic but remain consistent with later learning. Source
σ-bond: A covalent bond formed by the direct (end-to-end) overlap of atomic orbitals, resulting in electron density concentrated along the internuclear axis.
σ-bonds originate from overlapping orbitals such as sp³–sp³ (for C–C bonds) or sp³–1s (for C–H bonds). In alkanes, each carbon typically undergoes sp³ hybridisation, producing four equivalent hybrid orbitals arranged tetrahedrally to minimise electron-pair repulsion.
A key consequence of σ-bond formation is the resulting cylindrical symmetry of the electron density around the bond axis. This symmetry is responsible for the characteristic flexibility of alkanes.
Characteristics of σ-Bonds in Alkanes
Important features that arise from σ-bonding include:
High bond enthalpy, giving alkanes notable thermal and chemical stability.
Electron density concentrated directly between nuclei, creating strong, localised bonding interactions.
Lack of π-bonding, meaning no regions of electron density above or below the bond plane.
A rigid bond axis but a freely rotating bond orientation.
These properties contribute to the macroscopic inertness of alkanes, which depend heavily on the robustness of their σ-bonding framework.
Free Rotation Around C–C σ-Bonds
The OCR specification highlights that σ-bonds allow free rotation around the C–C bond. Because the electron density in a σ-bond is symmetrically distributed around the internuclear axis, rotation does not significantly disrupt orbital overlap.
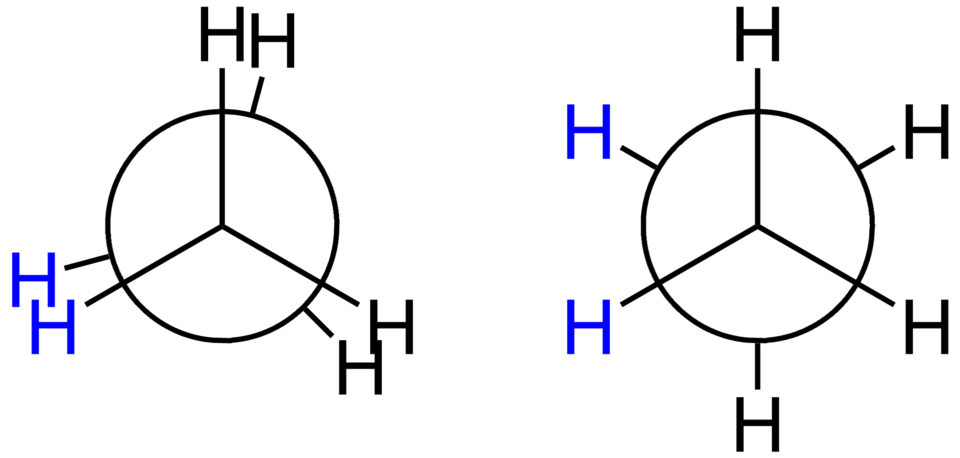
The image shows staggered and eclipsed conformations of ethane viewed along the C–C σ-bond axis. Rotation about this axis interconverts conformations; energetic differences are beyond this subsubtopic but link to later material. Source
Free rotation: The ability of atoms joined by a σ-bond to rotate about the bond axis without requiring bond breaking and with minimal energy change.
This free rotation is a defining feature of alkane conformations, permitting molecules to adopt numerous spatial arrangements. Although alkanes can rotate freely, some conformations are energetically more favourable than others due to repulsion between electron clouds of neighbouring bonds.
Consequences of Free Rotation
Free rotation affects both the physical and conceptual understanding of alkanes:
1. Conformational Diversity
Because rotation around C–C σ-bonds is unrestricted, alkanes possess an array of conformations — different spatial orientations of atoms caused solely by rotation.
Key points:
Conformations exist on a continuous spectrum, not as discrete isomers.
They interconvert rapidly under ambient conditions.
Conformations cannot be isolated in simple alkanes because rotation barriers are low.
2. No Stereochemical Fixedness
Unlike alkenes, which contain π-bonds that restrict rotation, alkanes cannot show E/Z stereoisomerism. The structural fluidity of σ-bonded frameworks prevents stable spatial restrictions around single bonds.
3. Influence on Physical Properties
Conformational flexibility plays a role in:
Intermolecular contact, and thus boiling points.
Packing efficiency in the solid state.
Shape and chain folding, particularly in longer alkanes.
Even though the specification does not require quantitative treatments of conformational energies, understanding how free rotation affects shape is essential to interpreting alkane properties more broadly.
Bonding Description at the Orbital Level
In alkanes, σ-bonds arise predominantly from sp³ hybrid orbitals. Each sp³-hybridised carbon forms four σ-bonds arranged approximately 109.5° apart.
sp³ hybridisation: The mixing of one s-orbital and three p-orbitals on a carbon atom to form four equivalent hybrid orbitals arranged tetrahedrally.
Normal sentence here so as not to place two definition blocks consecutively.
Visualising σ-Bonds and Rotation in Structural Representations
OCR expects students to understand how σ-bonds and free rotation are represented across different formula types. Recognition of these conventions is crucial for correctly interpreting exam questions involving structures and conformations.
Structural and Displayed Formulae
When drawing alkanes:
Displayed formulae show each σ-bond explicitly.
Structural formulae may condense long chains but still reflect single-bond connectivity.
Skeletal formulae, typically used for more complex molecules, imply σ-bonds at line ends and vertices.
Rotation around C–C bonds is not usually depicted directly but is inferred from the single-bond notation.
Three-Dimensional Representations
Because σ-bonds derive from sp³ hybrid orbitals, each carbon in an alkane adopts a tethedral arrangement. Common 3D representations include:
Wedge–dash diagrams, highlighting bonds coming out of or going behind the plane.
Newman projections, useful for visualising rotation around C–C bonds.
Sawhorse projections, offering an angled perspective of a bond axis.
These tools allow students to examine how rotation leads to different conformations and how the relative positions of substituents shift in space.
Summary of Key Points for OCR Study
Students must be confident with:
The nature of σ-bonds in alkanes and their formation through direct orbital overlap.
The consequence of cylindrical symmetry in σ-bonds leading to free rotation.
The relationship between σ-bond properties, conformational flexibility and alkane shape.
The difference between rotation-permitting σ-bonds and rotation-restricting π-bonds.
Recognising σ-bonds and conformational implications in different structural formula conventions.
These principles form the essential bonding framework upon which more advanced organic reactivity and mechanistic topics are built within the A-Level Chemistry course.
FAQ
Rotation around a sigma bond has a very low energy barrier because the electron density is symmetrically distributed along the bond axis. This means orbital overlap remains largely unchanged during rotation.
In contrast, rotation around a pi bond requires breaking the sideways overlap of p-orbitals, producing a high energy barrier. This is why pi bonds restrict rotation, unlike sigma bonds.
Newman projections allow the viewer to look directly along the carbon–carbon sigma bond, clearly displaying how substituents move as the bond rotates.
They highlight changes in torsional strain between conformations and make it easier to compare electron cloud repulsions, helping students visualise conformational preferences.
Although rotation is unrestricted, not all conformations have equal stability. Differences arise from torsional strain.
More stable conformations typically involve:
Reduced repulsion between bonding electron pairs
Staggered arrangements of substituents
Minimal overlap of electron clouds in adjacent C–H bonds
sp3 orbitals are highly directional, shaping electron density towards the region of bonding. Their tetrahedral arrangement maximises separation between electron pairs.
This geometry produces strong, localised sigma bonds and contributes to uniform bond lengths and angles in alkanes.
Free rotation gives long hydrocarbon chains considerable flexibility. This affects:
How chains pack in solids
Their ability to fold or coil
The overall rigidity or softness of a material
These structural variations influence melting points, viscosity, and mechanical properties, making conformational freedom crucial in understanding macroscopic behaviour.
Practice Questions
Explain what is meant by a sigma (σ) bond and why sigma bonds in alkanes allow free rotation.
(2 marks)
Sigma bond is formed by direct (end-to-end) overlap of orbitals. (1 mark)
Cylindrical symmetry of the sigma bond allows rotation around the C–C bond without breaking the bond. (1 mark)
Ethane, C2H6, contains only sigma bonds.
(a) Describe how the carbon–carbon sigma bond in ethane is formed.
(b) Explain why different conformations of ethane exist and why they cannot be isolated as separate compounds.
(c) State one way in which free rotation around sigma bonds influences the physical properties of longer-chain alkanes.
(5 marks)
(a)
Sigma bond formed by the head-on overlap of two sp3 hybrid orbitals on each carbon atom. (1 mark)
Electron density lies along the internuclear axis. (1 mark)
(b)
Rotation around the sigma bond produces different spatial arrangements (conformations). (1 mark)
Conformations cannot be isolated because rotation occurs freely and rapidly at room temperature due to a low energy barrier. (1 mark)
(c)
Free rotation allows chain flexibility, affecting intermolecular contact and therefore boiling point trends in longer alkanes. (1 mark)

