OCR Specification focus:
‘Describe halogenation by radical substitution under UV light with initiation, propagation and termination; explain limitations from further substitution and positional isomers.’
Alkanes undergo radical substitution when exposed to halogens under UV light, forming haloalkanes through a chain mechanism. This topic explores the steps and limitations of this reaction.
Radical Substitution: Core Principles
Radical substitution is a multi-step reaction pathway in which an alkane reacts with a halogen (e.g., chlorine or bromine) through a chain mechanism involving initiation, propagation, and termination. The process occurs because UV light provides the energy required to break halogen bonds and generate reactive species.
When first introduced, a radical must be defined.
Radical: A species with an unpaired electron, formed by homolytic bond fission and represented using a single dot.
Radicals are highly reactive because they seek electron pairing, allowing the chain mechanism to proceed rapidly once initiated.
Homolytic Fission and Radical Formation
The mechanism begins with homolytic fission, where a covalent bond splits evenly, producing two radicals. In radical substitution, the halogen molecule absorbs UV light, causing this symmetrical cleavage.
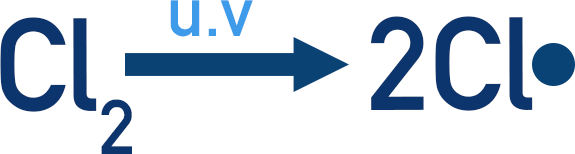
This image shows the homolytic fission of Cl2 under ultraviolet light, producing two Cl• radicals. It reinforces how UV initiates radical formation in the mechanism. Source
Homolytic fission: The equal splitting of a covalent bond to form two radicals, each retaining one electron from the shared pair.
Because homolytic fission requires energy input, radical substitution is photochemical and will not proceed in the dark. The high reactivity of radicals makes the steps that follow both fast and difficult to control.
A covalent bond’s energy is crucial to this process.
Bond enthalpy: The energy required to break one mole of a specified bond in the gas phase.
Strong C–H and C–C σ-bonds mean alkanes do not undergo many reactions, but once radicals form, the mechanism becomes self-sustaining.
Stages of Radical Substitution
Initiation
UV light breaks the halogen molecule (e.g., Cl₂ → 2Cl•) via homolytic fission. The formation of halogen radicals launches the chain mechanism.
Propagation
These steps allow the reaction to continue, generating products and regenerating radicals.
A halogen radical removes a hydrogen atom from the alkane:
Cl• + CH₄ → CH₃• + HClThe alkyl radical then reacts with another halogen molecule:
CH₃• + Cl₂ → CH₃Cl + Cl•
The regeneration of a halogen radical means propagation can repeat many times. This chain-like nature explains why even a small amount of UV light produces substantial substitution.
Termination
Termination occurs when radicals combine, removing reactive species from the mixture. Common termination steps include:
Cl• + Cl• → Cl₂
CH₃• + Cl• → CH₃Cl
CH₃• + CH₃• → C₂H₆
Since radicals are short-lived and highly reactive, any combination of two radicals is a termination event.
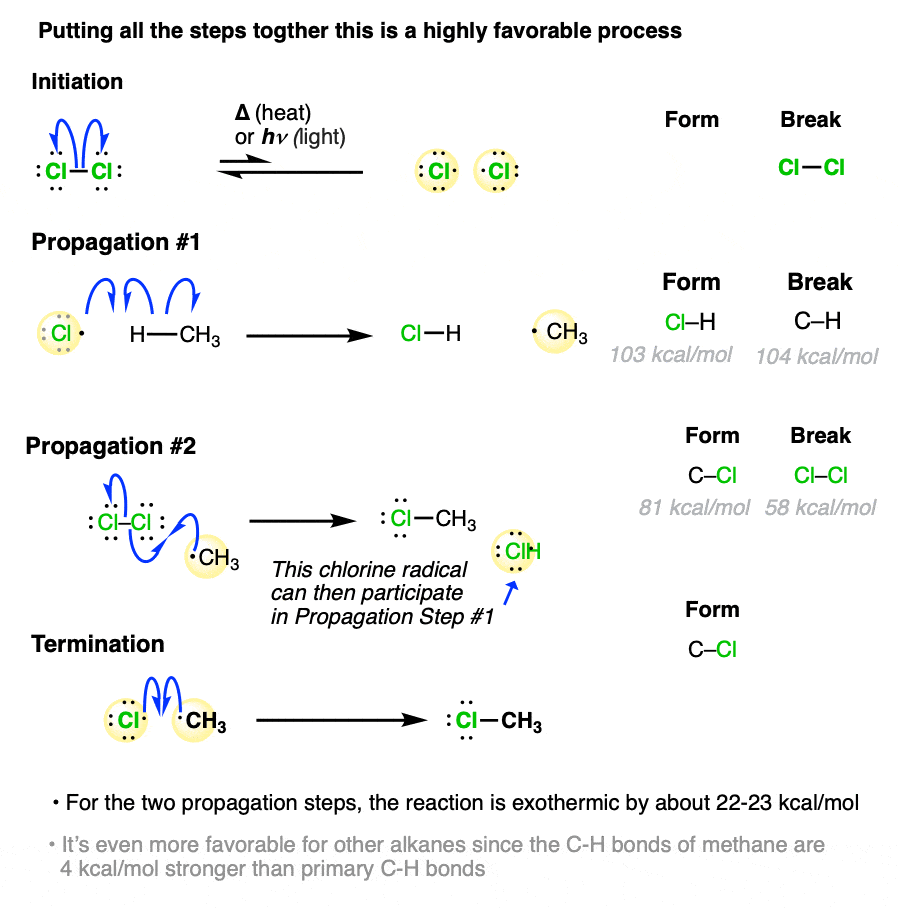
This figure shows the complete mechanism for free radical chlorination, illustrating initiation, propagation, and termination with clear structures and curly arrows consistent with A-Level expectations. Source
Limitations of Radical Substitution
Although often presented as a straightforward route to haloalkanes, radical substitution has significant limitations that restrict its synthetic usefulness.
1. Further Substitution
Once the first haloalkane (e.g., chloromethane) forms, the process does not stop. The product itself may undergo additional substitution, especially under prolonged UV exposure.
Possible sequential products include:
CH₃Cl → CH₂Cl₂ → CHCl₃ → CCl₄
Because each step also proceeds via radical pathways, mixtures of mono-, di-, tri-, and tetra-substituted compounds accumulate.
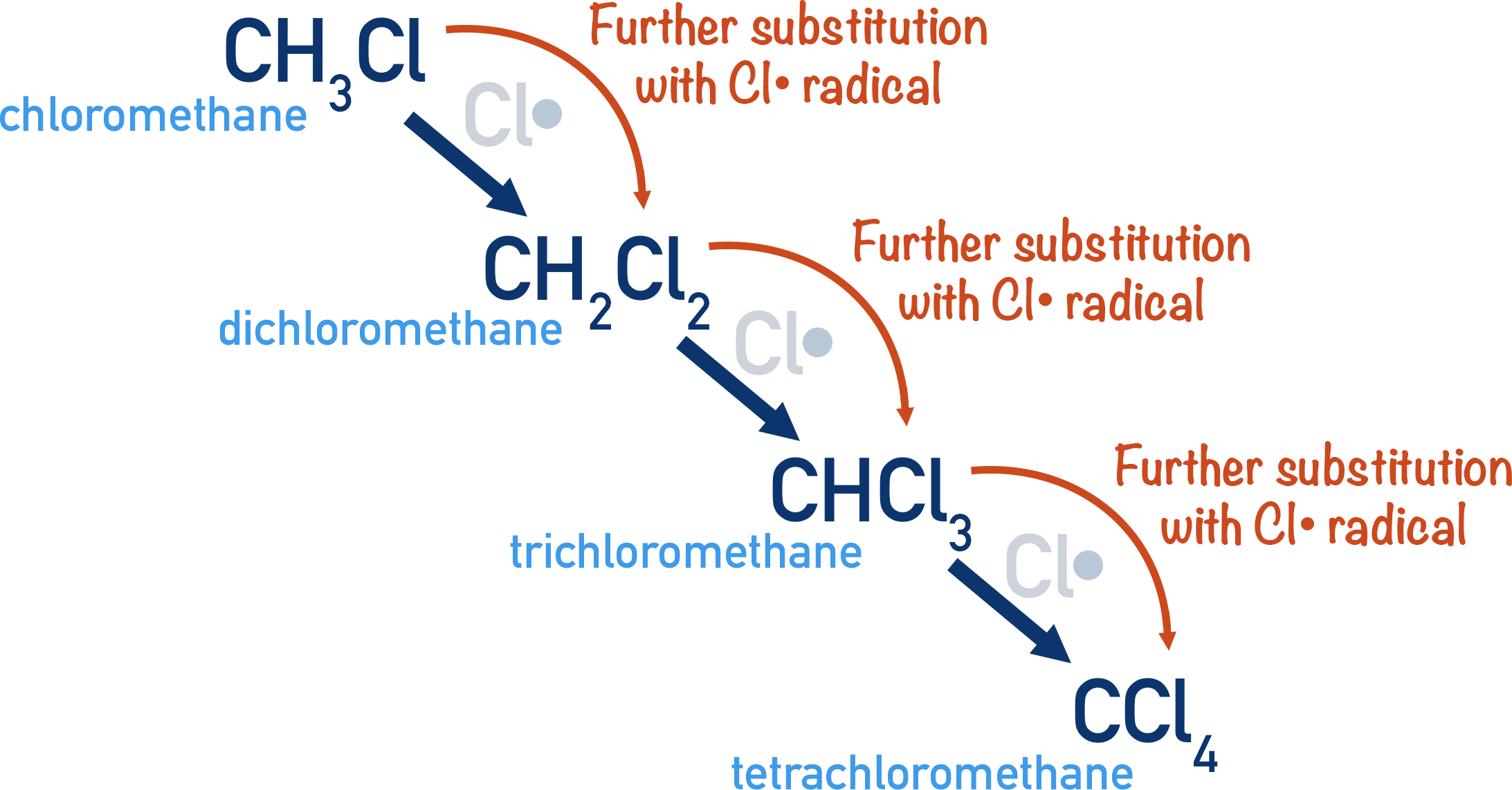
This image shows how chloromethane undergoes successive radical substitutions to form multiple chlorinated products, demonstrating why mixtures are inevitable in these reactions. Source
2. Positional Isomers
With alkanes longer than methane, substitution can occur at multiple positions along the carbon chain. Radicals do not significantly discriminate between equivalent hydrogen sites unless steric or stability factors intervene.
For example, substituting a hydrogen on propane may produce:
1-chloropropane (primary substitution)
2-chloropropane (secondary substitution)
The formation of positional isomers is a major limitation, as it leads to complex product mixtures requiring extensive separation.
After discussing positional variation, it is appropriate to define structural isomer.
Structural isomer: A compound with the same molecular formula as another but a different structural arrangement of atoms.
Because the mechanism generates radicals indiscriminately, both primary and secondary carbon sites may be substituted, depending on radical stability and reaction conditions.
Factors Affecting Selectivity and Product Distribution
Although radical substitution lacks precision, several factors influence the relative amounts of substituted products:
Radical Stability
More stable radicals (tertiary > secondary > primary) form more readily, increasing substitution at positions that generate the most stable intermediates.
Halogen Reactivity
Fluorination is explosive and largely uncontrollable.
Chlorination is moderately reactive and often yields mixtures.
Bromination is more selective because its propagation steps differ in activation energies.
Reaction Conditions
Higher temperatures or stronger UV sources increase further substitution.
Diluting the halogen or limiting exposure time can reduce over-substitution.
Using excess alkane limits the chances of a haloalkane being attacked again.
Why Radical Substitution Fits the Syllabus
The reaction demonstrates radical behaviour, chain mechanisms, and the challenges posed by uncontrolled reactivity. For OCR A-Level Chemistry, understanding the three mechanistic stages and appreciating the limitations of further substitution and positional isomer formation are essential for explaining why this reaction is more suitable for illustrating mechanisms than for precise organic synthesis.
FAQ
Radical substitution selectivity depends on the relative activation energies of the propagation steps. Chlorine has a moderate reactivity, so it often produces mixtures, whereas bromine’s slower hydrogen abstraction step favours formation of the most stable radical, resulting in higher selectivity.
Fluorine reacts too rapidly for selectivity to develop, while iodine’s reaction is endothermic and does not proceed efficiently.
Higher temperatures increase the rate of both propagation and further substitution, leading to a greater proportion of poly-substituted products.
At lower temperatures, termination becomes more competitive, reducing the overall number of substitution cycles.
The key factor is radical stability. A tertiary radical is more stable due to greater alkyl group electron-donating effects.
This increased stability lowers the activation energy for abstraction of a tertiary hydrogen, making substitution at that carbon far more favourable.
Yes. Several strategies can reduce side products:
Using excess alkane to minimise further substitution of the haloalkane.
Limiting UV exposure to shorten the chain reaction.
Lowering halogen concentration to reduce immediate re-attack.
These techniques cannot eliminate mixtures entirely but can improve the proportion of desired product.
The reaction provides poor control over regiochemistry and degree of substitution, producing complex mixtures.
Additionally, radicals generated during the process can react with many functional groups, risking unwanted degradation or side reactions.
These limitations make the method unsuitable for precise multi-step synthesis despite its mechanistic value.
Practice Questions
Chlorine reacts with methane in the presence of UV light to form chloromethane.
(a) State the type of bond fission that occurs in the initiation step.
(b) Explain why UV light is required for this reaction to take place.
(2 marks)
(a) (1 mark)
Homolytic fission.
(b) (1 mark)
UV light provides the energy to break the Cl–Cl bond and generate chlorine radicals.
Halogenation of propane by radical substitution produces a mixture of haloalkanes.
(a) Outline the two propagation steps in the radical substitution mechanism of propane with chlorine.
(b) Explain why this reaction produces both 1-chloropropane and 2-chloropropane.
(c) State one limitation of radical substitution that reduces its usefulness in organic synthesis (other than positional isomer formation).
(5 marks)
(a) (2 marks)
Award 1 mark for each correct propagation step:
Cl• removes H from propane to form HCl and a propyl radical.
Propyl radical reacts with Cl2 to form chloropropane and another Cl•.
(b) (2 marks)
Award up to 2 marks for:
Radical attack can occur at different positions on the propane molecule.
Substitution at a primary carbon gives 1-chloropropane; substitution at the secondary carbon gives 2-chloropropane.
(c) (1 mark)
Any one of:
Further substitution occurs, producing di-, tri- or tetra-chlorinated products.
Reaction produces complex mixtures that are difficult to separate.

