OCR Specification focus:
‘Describe complete combustion of alkanes as fuels and risks of incomplete combustion in limited oxygen, including formation and dangers of carbon monoxide.’
Alkanes are widely used fuels whose combustion behaviour is essential in understanding energy production, pollutant formation, and safety. Their reactions with oxygen illustrate key redox principles and environmental implications.
Combustion of Alkanes
Alkanes are saturated hydrocarbons that react with oxygen in exothermic combustion processes. Their high C–H and C–C σ-bond enthalpies provide substantial energy output when oxidised. Combustion may occur as complete or incomplete combustion depending on oxygen availability, and understanding both pathways is central to their chemical behaviour and real-world applications as fuels.
Complete Combustion
Complete combustion occurs when an alkane burns in excess oxygen, producing only carbon dioxide (CO₂) and water (H₂O). This process releases significant thermal energy because oxidation is extensive and the products are at a low energy state relative to the reactants.
Complete combustion: The oxidation of a fuel in plentiful oxygen, producing only carbon dioxide and water.
A normal sentence must appear here to separate definition and other required blocks, ensuring clarity and continuity of explanation.
The general reaction for complete combustion depends on the alkane formula CₙH₂ₙ₊₂, and the stoichiometry reflects full oxidation of both carbon and hydrogen atoms.
Complete Combustion of an Alkane
CₙH₂ₙ₊₂ + (1.5n + 0.5) O₂ → nCO₂ + (n + 1)H₂O
CₙH₂ₙ₊₂ = Alkane with n carbon atoms
O₂ = Oxygen gas, reactant
CO₂ = Carbon dioxide, oxidation product
H₂O = Water, oxidation product
Burning alkanes completely is a key feature that makes them efficient fuels in domestic heating, industrial boilers, and internal combustion engines. Adequate oxygen supply enables cleaner flame characteristics and minimises toxic by-products.
Balanced combustion of methane showing reactant and product molecules in their correct stoichiometric ratios. The diagram highlights complete oxidation producing carbon dioxide and water. It also illustrates conservation of atoms, offering slightly more detail than the syllabus requires. Source
Incomplete Combustion
Incomplete combustion occurs when oxygen availability is restricted. This leads to the formation of carbon monoxide (CO), carbon (soot) or both, along with water. Students should understand why restricted oxygen prevents all carbon atoms from reaching their highest oxidation state.
Incomplete combustion: The oxidation of a fuel in limited oxygen, producing carbon monoxide and/or carbon instead of carbon dioxide.
A normal sentence must appear here to maintain spacing requirements and avoid back-to-back definition or equation blocks.
Incomplete combustion is highly relevant in household and industrial safety because the gaseous product carbon monoxide is physiologically dangerous.
Carbon Monoxide Formation and Its Hazards
Carbon monoxide (CO) is a highly toxic, colourless, and odourless gas formed when carbon is only partially oxidised. It binds strongly to haemoglobin, preventing effective oxygen transport in blood. Even low concentrations can cause severe poisoning, making awareness of ventilation, combustion conditions, and detector use critically important.
Carbon monoxide: A toxic gas formed by partial oxidation of carbon during incomplete combustion, capable of binding to haemoglobin and impairing oxygen transport.
Students should recognise that CO formation is unavoidable when oxygen is insufficient, particularly in enclosed spaces or poorly maintained appliances such as gas heaters and boilers.
Conditions Influencing Combustion Pathways
The balance between complete and incomplete combustion depends on several factors:
Oxygen availability: The primary determinant of oxidation extent.
Flame temperature: Higher temperatures promote complete combustion.
Mixing of fuel and air: Turbulence and good airflow improve oxidation efficiency.
Hydrocarbon chain length: Larger alkanes require more oxygen and may produce more soot if combustion is inefficient.
These factors interplay to determine the combustion products observed in practical situations, especially in engines and domestic gas systems.
Environmental and Practical Implications
Complete combustion produces carbon dioxide, contributing to the global atmospheric carbon pool. Although cleaner than incomplete combustion, CO₂ is a greenhouse gas, making combustion control an environmental priority. Students should recognise that understanding the chemistry helps inform energy policy and engineering design decisions aimed at reducing emissions.
Incomplete combustion, in contrast, contributes to:
Toxic CO emissions
Soot particles, which can cause respiratory irritation and contribute to particulate air pollution
Reduced energy efficiency, since carbon is not fully oxidised
Managing combustion conditions effectively reduces these negative impacts.
Visual and Practical Indicators
Real-world observations can help students relate theory to practice:
Complete combustion often produces a blue, clean flame with minimal residue.
Incomplete combustion commonly produces a yellow, sooty flame, as glowing carbon particles radiate visible light.
These indicators, although qualitative, are widely used in laboratory and industrial settings to assess combustion efficiency.

Photograph of Bunsen burner flames showing how increasing air supply changes the flame from yellow and luminous to blue and non-luminous. Yellow flames indicate incomplete combustion, while blue flames indicate more complete combustion. The labelled air-valve positions provide context beyond the syllabus but aid understanding. Source
Why Alkanes Burn Readily as Fuels
Although alkanes are generally chemically unreactive due to low bond polarity and high σ-bond enthalpy, combustion is feasible because the reaction with oxygen is strongly exothermic and self-sustaining once initiated. This makes alkanes major components of fuels such as natural gas, petrol, diesel, and LPG.
Safety Considerations
Key precautions relate to ensuring complete combustion wherever possible to minimise CO formation:
Maintaining proper ventilation in enclosed spaces
Using well-serviced appliances to ensure optimal air–fuel mixing
Installing carbon monoxide detectors
Avoiding blocked flues or chimneys
Because CO is undetectable by human senses, engineered safety controls are essential.
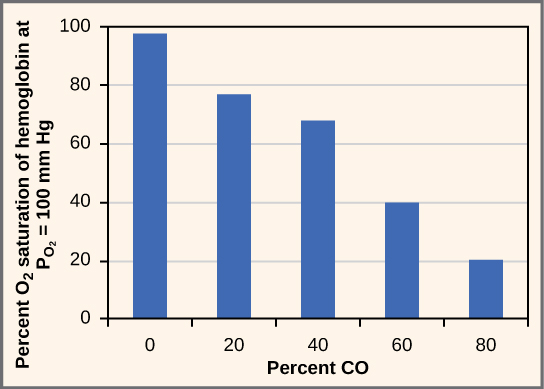
Bar chart showing how oxygen saturation of haemoglobin declines as carbon monoxide levels increase. This illustrates why CO produced during incomplete combustion is dangerous. The quantitative axis values exceed syllabus requirements but reinforce the key physiological effect. Source
Summary of Key Points for OCR A-Level Chemistry
These notes emphasise specification requirements:
Complete combustion produces CO₂ and H₂O.
Incomplete combustion in limited oxygen produces CO and/or carbon.
Carbon monoxide is toxic and poses serious health dangers.
Understanding combustion outcomes links molecular behaviour to fuel performance and safety.
FAQ
Alkanes with longer carbon chains require more oxygen for complete combustion, increasing the likelihood of incomplete combustion if oxygen supply is restricted.
Branching reduces surface contact between molecules, lowering boiling points and improving vapour mixing with air. This can enhance combustion efficiency by promoting more uniform oxidation.
Highly branched alkanes therefore tend to burn more cleanly than their straight-chain isomers.
Soot forms when carbon atoms are not fully oxidised. Alkanes with higher carbon content release more carbon per mole, increasing soot formation under limited oxygen.
Soot formation is enhanced by:
Poor mixing of fuel and air
Low flame temperature
Longer, unbranched hydrocarbon chains
Branched alkanes and gaseous alkanes generally produce less soot.
A yellow flame results from glowing carbon particles produced during incomplete combustion, indicating restricted oxygen supply.
A blue flame forms when combustion is more complete and the air–fuel mixture is well-oxygenated.
Flame appearance is influenced by:
Air intake settings
Burner design
Temperature and turbulence within the flame
Ventilation ensures a sufficient supply of oxygen for complete combustion. Inadequate airflow reduces oxygen concentration, increasing carbon monoxide formation.
Airflow patterns affect how efficiently fuel mixes with oxygen. Dead zones or obstructions can create pockets of incomplete combustion even when total room oxygen seems adequate.
Modern appliances incorporate controlled air inlets to maintain consistent mixing and minimise CO production.
Carbon monoxide binds irreversibly to haemoglobin far more strongly than oxygen, preventing oxygen transport even at low concentrations.
In enclosed spaces, CO accumulates because it is undetectable by smell or sight, allowing dangerous levels to build without triggering human sensory warning systems.
By contrast, carbon dioxide generally causes discomfort at increasing levels, prompting ventilation before lethal concentrations are reached.
Practice Questions
Propane can undergo both complete and incomplete combustion.
(a) Write the products of the complete combustion of propane.
(b) State one product formed during incomplete combustion and explain why it forms.
(2 marks)
(a)
Carbon dioxide and water (1 mark)
(b)
Identifies carbon monoxide or carbon (soot) as a product (1 mark)
Explains it forms due to limited oxygen supply preventing full oxidation (1 mark)
(Max 2 marks)
Explain why carbon monoxide is dangerous and describe how its formation is related to combustion conditions in domestic appliances. Your answer should refer to:
the process of incomplete combustion
the behaviour of carbon monoxide in the body
the role of ventilation and appliance maintenance.
(5 marks)
Award marks for any five of the following points:
Incomplete combustion occurs when oxygen supply is insufficient (1 mark)
Carbon monoxide forms because carbon atoms are only partially oxidised (1 mark)
Carbon monoxide binds strongly to haemoglobin (1 mark)
This reduces oxygen transport around the body, leading to hypoxia (1 mark)
Poor ventilation or poorly maintained appliances increase the likelihood of incomplete combustion and CO production (1 mark)
CO is colourless and odourless, making it hard to detect without safety devices (1 mark)
(Max 5 marks)

