OCR Specification focus:
‘Define stereoisomers, E/Z isomerism from restricted rotation and different substituents at each carbon; relate cis–trans as a special case; assign E/Z using CIP priority rules.’
Stereoisomerism in alkenes is central to understanding structural variation in organic molecules. Restricted rotation around C=C bonds creates distinct spatial arrangements with meaningful chemical and physical consequences.
Stereoisomerism in Alkenes
Stereoisomers are molecules with the same structural formula but different spatial arrangement of atoms due to restricted rotation around a C=C double bond. This restriction arises because the π-bond prevents free rotation, locking substituents in fixed positions.
Stereoisomers: Compounds with identical structural formulae but differing spatial arrangement of atoms.
A single sentence separates this from further defined material. Understanding how these arrangements form is essential for recognising distinct isomers and assigning correct names using IUPAC rules.
The double bond of an alkene consists of one σ-bond and one π-bond; it is the π-bond that produces rigidity. Therefore, stereoisomerism occurs only when each carbon in the double bond is bonded to two different groups.
E/Z Isomerism: Core Principles
E/Z isomerism is the formal system used to describe geometric arrangements around a C=C bond. It becomes necessary when substituents are not identical and requires applying the Cahn–Ingold–Prelog (CIP) priority rules.
E/Z Isomerism: A type of stereoisomerism arising from restricted rotation around C=C bonds where different substituents create distinct spatial arrangements.
This system avoids ambiguity by providing a universal naming method independent of common naming conventions.
CIP Priority Rules
CIP rules determine which substituent on each carbon of the double bond has higher priority. Higher atomic number means higher priority. Once priorities are assigned:
Z (zusammen): Higher-priority groups are on the same side of the double bond.
E (entgegen): Higher-priority groups are on opposite sides of the double bond.
Bullet points enhance stepwise clarity:
Identify the atoms directly attached to each carbon of the C=C.
Assign priority based on atomic number (higher = higher priority).
If first atoms are identical, move outward along the substituent chain until a difference is found.
Compare the relative positions of the highest-priority substituents across the double bond.
Assign E or Z accordingly.
The E and Z descriptors use the Cahn–Ingold–Prelog (CIP) priority rules to compare the two substituents on each carbon of the double bond.
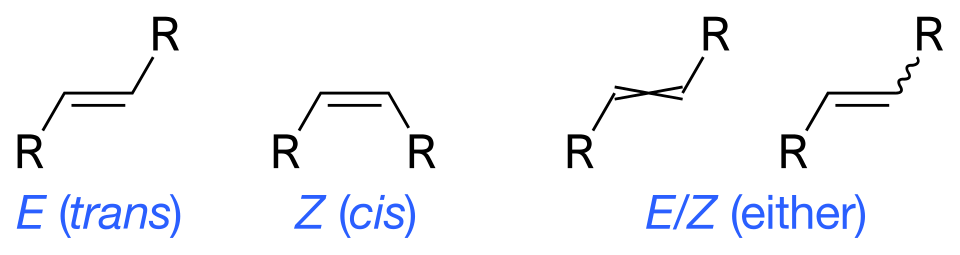
Skeletal formulas demonstrating how alkene stereochemistry is labelled using E/Z notation. High-priority substituents on the same side correspond to the Z isomer, while opposite-side priorities correspond to the E isomer. Generic R groups represent substituents without adding content beyond syllabus requirements. Source
Why Restricted Rotation Matters
The presence of the π-bond prevents rotation, meaning these arrangements cannot interconvert without breaking the π-bond. This makes E/Z isomers structurally distinct and often physically different in properties such as boiling point and dipole moment.
Each carbon in a C=C is trigonal planar, and restricted rotation about the double bond fixes substituents in space, giving rise to stereoisomers.
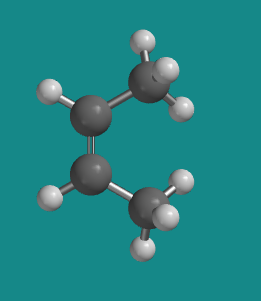
Ball-and-stick model of cis‑2‑butene showing both methyl groups positioned on the same side of the C=C bond. The model highlights planar geometry and fixed substituent arrangement resulting from restricted rotation. No content beyond geometric illustration is included. Source
Conditions for E/Z Isomerism
E/Z stereoisomerism requires the following:
Both carbons in the C=C must have two different substituents.
The substituents must be comparable using CIP rules.
The double bond must not be part of a system that inherently prevents distinction, such as identical substituent pairs.
If either carbon carries two identical groups, stereoisomerism cannot occur.
Cis–Trans Isomerism as a Special Case
While E/Z terminology is systematic, cis–trans isomerism is a simpler, older naming system used only when substituents meet specific structural conditions.
Cis–trans Isomerism: A form of stereoisomerism where identical or clearly defined substituent groups are on the same (cis) or opposite (trans) sides of a C=C bond.
A single sentence here ensures compliance with spacing rules. In cases where cis–trans terminology is applicable, E/Z naming is still valid and preferred for precision.
In cis isomers, the two identical or comparable groups are on the same side of the double bond, while in trans isomers they are on opposite sides.
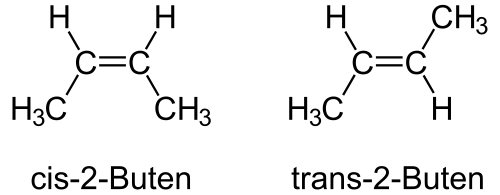
Structural formulas of cis‑2‑butene and trans‑2‑butene, showing identical groups arranged on the same side (cis) or opposite sides (trans) of the C=C bond. The rigidity of the π‑bond prevents interconversion. No additional material beyond geometric comparison is included. Source
When Cis–Trans Is Not Sufficient
Many alkenes have substituents that cannot be categorised simply as “same side” or “opposite side” due to structural complexity. CIP ranking removes this ambiguity. For instance:
If substituent groups differ, cis–trans terminology becomes ambiguous.
E/Z classification still applies because it assigns priority and compares the highest-ranked substituents.
Visualising Spatial Arrangement
To interpret E/Z and cis–trans forms effectively, organic chemists typically use:
Displayed formulae, showing the arrangement of substituents around the double bond.
Skeletal formulae, common for more complex structures.
3D wedge–dash representations, useful for distinguishing substituent orientation relative to the plane of the double bond.
Each representation helps clarify the distinction between isomers without rotating the molecule.
Syllabus-Focused Key Features
The OCR specification emphasises:
The need to define stereoisomers clearly in the context of spatial arrangement.
Recognition that restricted rotation around the C=C bond is essential for E/Z formation.
Understanding that cis–trans isomerism is a special case of E/Z when identical substituents are present.
Application of CIP priority rules is required to assign E or Z configurations systematically.
Importance in Organic Chemistry
Stereoisomerism affects chemical behaviour and physical properties. Identifying E/Z or cis–trans forms allows predictions about:
Intermolecular forces
Dipole orientation
Biological activity
Reactivity trends
These considerations highlight why mastering E/Z and cis–trans nomenclature is essential for accurate structural descriptions in organic chemistry.
Summary of Key Points (Bullet List for Recall)
Stereoisomers differ in spatial arrangement due to π-bond restricted rotation.
E/Z isomerism uses CIP rules to assign priority and determine arrangement.
Cis–trans applies only when identical groups allow simple comparison.
Cis = same side, Trans = opposite sides.
E/Z terminology is universally applicable and avoids ambiguity.
FAQ
E/Z stereoisomerism is impossible when either carbon of the double bond is bonded to two identical groups. This removes the difference needed for priority assignment.
Other features that prevent E/Z isomerism include:
C=C bonds within symmetrical rings where substituent distinction is lost
Conjugated or cumulative systems where substituents effectively match in priority
Terminal alkenes with two hydrogens on one carbon
These indicators help eliminate unnecessary stereochemical analysis early.
E/Z designations depend only on substituents directly attached to the double-bonded carbons. Chiral centres elsewhere do not influence substituent priority unless they alter the immediate atomic sequence used in CIP comparison.
A molecule can therefore simultaneously display:
E/Z stereoisomerism at the alkene
Optical isomerism at one or more chiral centres
Each type of stereochemistry is evaluated independently.
When the first atoms attached to the C=C are the same, CIP rules require comparison of the next atoms along each substituent chain.
The correct approach is:
Identify all atoms one bond further from the double bond
Arrange these atoms in order of descending atomic number
Compare sets until the first point of difference appears
This systematic method ensures consistent priority decisions even in highly branched structures.
In small and medium-sized rings, substituents fixed above or below the plane of the ring retain a meaningful “same side” or “opposite side” relationship.
Cis–trans terminology works when:
Each carbon of the C=C bears substituents whose relative spatial orientation is clearly defined by the ring
The ring prevents rotation, locking substituents into distinguishable positions
However, for large rings where geometry becomes flexible, cis–trans becomes unreliable and E/Z notation is preferred.
Restricted rotation can be overcome only by providing enough energy to break the π-bond, typically via high-energy photochemical processes or thermal excitation.
Once the π-bond is broken:
Rotation can occur freely
E/Z configuration may scramble
Re-formed C=C bonds may produce mixtures of stereoisomers
In standard conditions relevant to organic reactions, rotation does not occur, preserving stereochemical integrity.
Practice Questions
But-2-ene can exist as two stereoisomers.
(a) State the type of stereoisomerism shown by but-2-ene.
(b) Explain why but-1-ene does not show this type of stereoisomerism.
(2 marks)
(a)
1 mark: Identifies E/Z or cis–trans stereoisomerism.
(b)
1 mark: But-1-ene has two identical groups (two hydrogens) on one carbon of the C=C.
1 mark: Therefore, it cannot form stereoisomers because each carbon must have two different groups.
2-bromo-2-butene is shown below.
CH3–C(Br)=C(H)–CH3
(a) Assign E or Z configuration to this alkene.
(b) Explain in detail how the Cahn–Ingold–Prelog (CIP) priority rules are used to determine this stereochemical assignment.
(c) State why E/Z notation is preferred over cis–trans terminology for this molecule.
(5 marks)
(a)
1 mark: Correct assignment: E (higher-priority groups on opposite sides).
(Allow Z only if the student’s explanation in part (b) matches the alternative drawing they assumed.)
(b)
Award up to 3 marks:
1 mark: Identifies the two groups on each carbon of the C=C.
1 mark: Correctly states that priority is based on atomic number (Br > CH3 and CH3 > H).
1 mark: Correctly concludes that the highest-priority groups are on opposite sides, giving the E configuration.
(c)
1 mark: Cis–trans notation is not suitable because the substituents are not pairs of identical groups or are too complex for simple comparison.
1 mark: E/Z notation gives an unambiguous description based on CIP priorities.

