OCR Specification focus:
‘Determine whether a structure can show E/Z or cis–trans isomerism from its substituents and double bond arrangement; apply CIP priorities where required.’
Alkenes can display stereoisomerism because restricted rotation around the C=C double bond fixes substituents in position. Understanding when stereoisomers arise is essential for predicting structural possibilities and avoiding ambiguous structural representations.
Understanding When E/Z Isomerism Occurs
Stereoisomerism in alkenes arises when rotation around the C=C double bond is restricted, meaning different spatial arrangements become possible. For OCR A-Level Chemistry, identifying potential E/Z isomers depends on analysing substituents attached to each carbon of the double bond.
Before assessing structures, recall the key term required for this process.
Stereoisomers: Compounds with the same structural formula but different arrangements of atoms in space.
To identify whether a molecule can exhibit E/Z isomerism, it is essential to examine substituent variety on each carbon of the double bond.
Conditions Required for E/Z Isomerism
An alkene can show E/Z stereoisomerism only when both carbons of the C=C bond have two different substituents. If either carbon carries two identical groups, E/Z isomerism is impossible.
Key conditions:
The double bond must be C=C, creating restricted rotation due to the presence of a π-bond.
Each carbon in the double bond must be bonded to two different groups.
CIP (Cahn–Ingold–Prelog) priority rules must be used to assign E/Z when more than simple identical/different comparisons are required.
Understanding cis–trans as a Special Case
Cis–trans terminology applies only to certain E/Z isomers. It is used when each carbon of the double bond has one substituent that is the same on both sides. The arrangement is then described as cis (same side) or trans (opposite sides). However, many alkenes require full CIP rule application because substituents may not match or may be complex.
For a simple disubstituted alkene such as but-2-ene, cis–trans isomerism arises because each carbon of the C=C is bonded to two different groups.
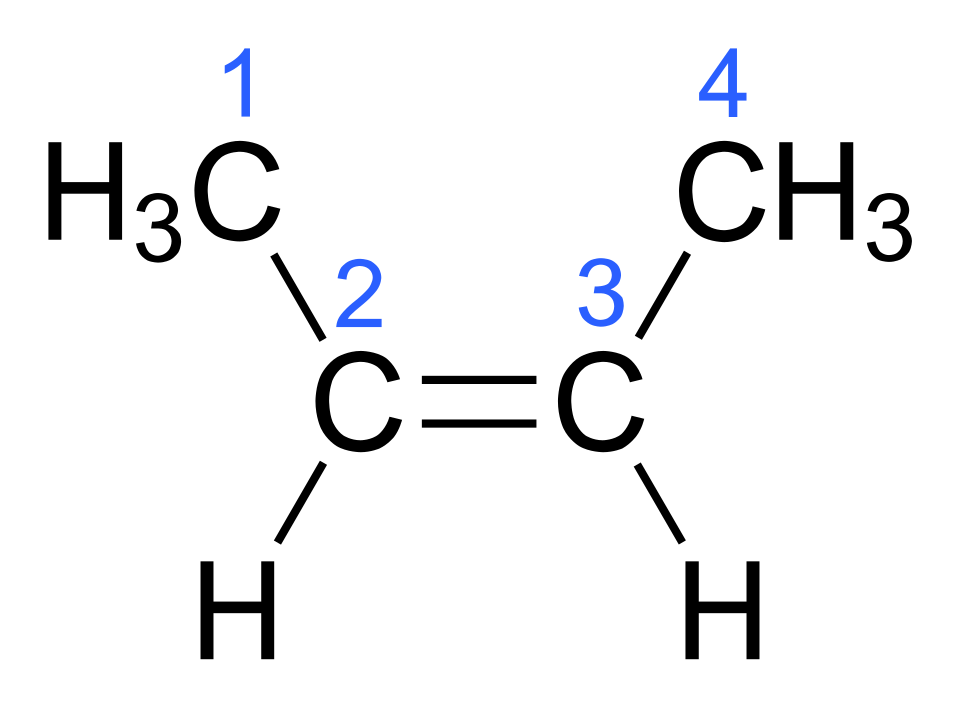
Skeletal formula of cis-2-butene, showing both CH₃ groups on the same side of the C=C bond. This diagram illustrates how restricted rotation around the double bond leads to fixed “cis” geometry. It focuses only on the connectivity and spatial arrangement relevant to cis–trans isomerism at A-level. Source
A normal explanatory sentence is required here before CIP rules are introduced, helping establish context for their use in stereochemical analysis.
CIP Priority Rules: A system that ranks substituents based on atomic number to determine priority for assigning E or Z stereochemistry.
The OCR specification emphasises applying these rules when a simple visual comparison is insufficient.
Applying CIP Rules to Determine E/Z Configuration
CIP priority assignment follows a structured approach to determine which substituent on each carbon has higher priority. Once identified, the positions of the higher-priority groups relative to the double bond define the stereoisomerism:
Z (zusammen): Higher-priority groups on the same side
E (entgegen): Higher-priority groups on opposite sides
Procedure for Identifying E/Z
Students should learn a clear, repeatable sequence for evaluating structures.
Step-by-step process:
Locate the C=C bond and mark the two carbons.
Identify the two substituents attached to each carbon.
Apply CIP rules to determine which substituent on each carbon has higher priority.
Compare the positions of the higher-priority substituents:
Same side → Z isomer
Opposite sides → E isomer.
Once the highest-priority group on each carbon has been identified using the CIP rules, the alkene is Z if these groups are on the same side and E if they are on opposite sides.
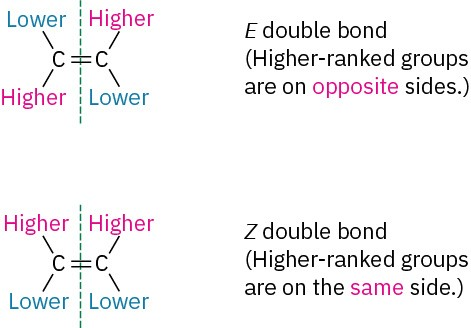
Schematic comparison of an E and a Z double bond, with each substituent marked as higher or lower priority. The diagram highlights that E isomers have higher-priority groups on opposite sides of the double bond, whereas Z isomers have them on the same side. The image includes a brief explanatory label but no extra chemistry beyond the E/Z definition needed at this level. Source
One sentence must appear here before introducing any further block formatting to maintain correct structure.
Restricted Rotation: The inability of atoms around a double bond to rotate freely due to the presence of a π-bond.
Determining Whether Stereoisomerism Is Possible at All
Identifying possible E/Z stereoisomers involves not only naming the isomer type but recognising if stereoisomerism can occur in the first place. This aligns directly with OCR expectations.
Conditions to Check for Possibility
Use the following to assess whether a double bond can give rise to stereoisomers:
Check substituent variety on each carbon
Different groups → potential E/Z
Identical groups → no stereoisomerism
Confirm restricted rotation by the presence of the C=C
Consider ring systems such as cycloalkenes
Rings constrain substituents; cis–trans descriptions may apply if substituent positions differ.
When E/Z Isomerism Cannot Occur
An alkene cannot show stereoisomerism when:
One carbon of the double bond has two identical substituents
The molecule contains a terminal double bond with identical substituents on the same carbon
The double bond is within a structure where substituent positions do not permit distinguishable spatial arrangements.
A further clarifying sentence should remain here to separate conceptual blocks and reinforce understanding of structural limitations.
Visual and Structural Assessment Strategies
Students should be confident analysing skeletal, displayed, or structural formulae to determine stereochemical possibilities. For OCR exams, this requires interpreting implicit hydrogens and substituent patterns.
Helpful techniques:
Highlight substituents on each carbon of the C=C bond before comparing.
Redraw complex molecules around the double bond region to simplify priority determination.
Check for symmetry, as symmetrical structures often eliminate E/Z potential.
Relate back to general rules, ensuring each carbon bears non-identical groups.
Bullet-Point Review of Key Identification Skills
Identify the C=C bond clearly.
Confirm restricted rotation.
Ensure each carbon has two different groups.
Apply CIP priority rules where needed.
Decide if substituent arrangement creates distinguishable spatial forms.
Conclude whether E/Z isomerism is possible from the structure.
A brief sentence here reinforces the conceptual unity of these skills before ending the notes.
These points align directly with the specification requirement to determine whether a structure can show E/Z or cis–trans isomerism and the essential ability to apply CIP priorities appropriately.
FAQ
Cis–trans notation is only appropriate when each carbon of the C=C bond has one substituent that is identical across the double bond. This usually applies to simple disubstituted alkenes such as but-2-ene.
For alkenes with complex or different substituents, E/Z notation must be used because it follows the CIP priority rules, ensuring an unambiguous description of the stereochemistry.
CIP priority depends on atomic number, not total atomic mass or group size. A smaller group containing a higher atomic number atom may outrank a bulkier group.
If the first atoms are identical, the sequence rules extend outward along the chain until a point of difference is found.
Cyclic systems can exhibit cis–trans relationships due to the restricted rotation of ring structures, but this is not classified as E/Z stereoisomerism.
E/Z terminology specifically applies to double bonds, whereas cis/trans in rings refers to relative positions above or below the ring plane.
CIP rules require comparing atoms stepwise along each substituent until a difference appears.
If necessary, branches are treated as sets of atoms arranged in decreasing priority, ensuring a consistent method for distinguishing substituents with similar beginnings.
Resonance does not change the basic requirement that each carbon of the double bond must have two different substituents.
However, delocalised systems such as conjugated alkenes may limit the ability to rotate or isolate individual double bonds, sometimes preventing distinct stereoisomers from being isolated or observed.
Practice Questions
Explain why the alkene CH3CH=CHCH3 can exist as stereoisomers, but CH2=CHCH3 cannot.
(2 marks)
CH3CH=CHCH3 can show E/Z stereoisomerism because each carbon in the C=C bond has two different substituents. (1 mark)
CH2=CHCH3 cannot show stereoisomerism because one of the carbons in the double bond has two identical substituents (two H atoms). (1 mark)
Figure 1 shows an alkene with substituents labelled A, B, C, and D attached to the two carbons of the C=C bond.
Using the CIP priority rules:
a) State the condition required for an alkene to show E/Z stereoisomerism. (1 mark)
b) Assign priorities to substituents A and B on carbon 1 and to substituents C and D on carbon 2. Justify your assignments. (2 marks)
c) Deduce whether the alkene shown is the E or the Z isomer. Explain your reasoning. (2 marks)
(5 marks)
a)
An alkene shows E/Z stereoisomerism when each carbon of the C=C bond is attached to two different substituents. (1 mark)
b)
Correct assignment of higher priority substituent on carbon 1 using atomic number comparison (e.g., A over B if A has an atom of higher atomic number). (1 mark)
Correct assignment on carbon 2 using the same CIP logic (C vs D). (1 mark)
c)
Correctly identifying whether highest-priority groups lie on the same side (Z) or opposite sides (E). (1 mark)
Clear explanation that the E isomer has higher-priority substituents on opposite sides; the Z isomer has them on the same side. (1 mark)
If you want versions with diagrams, multiple-choice options, or greater difficulty, I can create those too.

