OCR Specification focus:
‘Explain alkenes’ high reactivity via lower π-bond enthalpy; summarise additions with H₂/Ni to alkanes, X₂ to dihaloalkanes, HX to haloalkanes, and steam/H₃PO₄ to alcohols.’
Alkenes show distinctive reactivity due to the nature of their π-bond, which is weaker than a σ-bond and therefore easily broken, enabling a range of important addition reactions.
Reactivity of Alkenes and the Role of the π-Bond
The reactivity of alkenes stems from the behaviour of the C=C double bond, which contains both a σ-bond and a π-bond. The π-bond forms from the sideways overlap of adjacent p-orbitals, creating electron density above and below the plane of the carbon atoms. This arrangement means that the π-bond is weaker and more exposed than a σ-bond, making alkenes far more reactive than the corresponding alkanes.
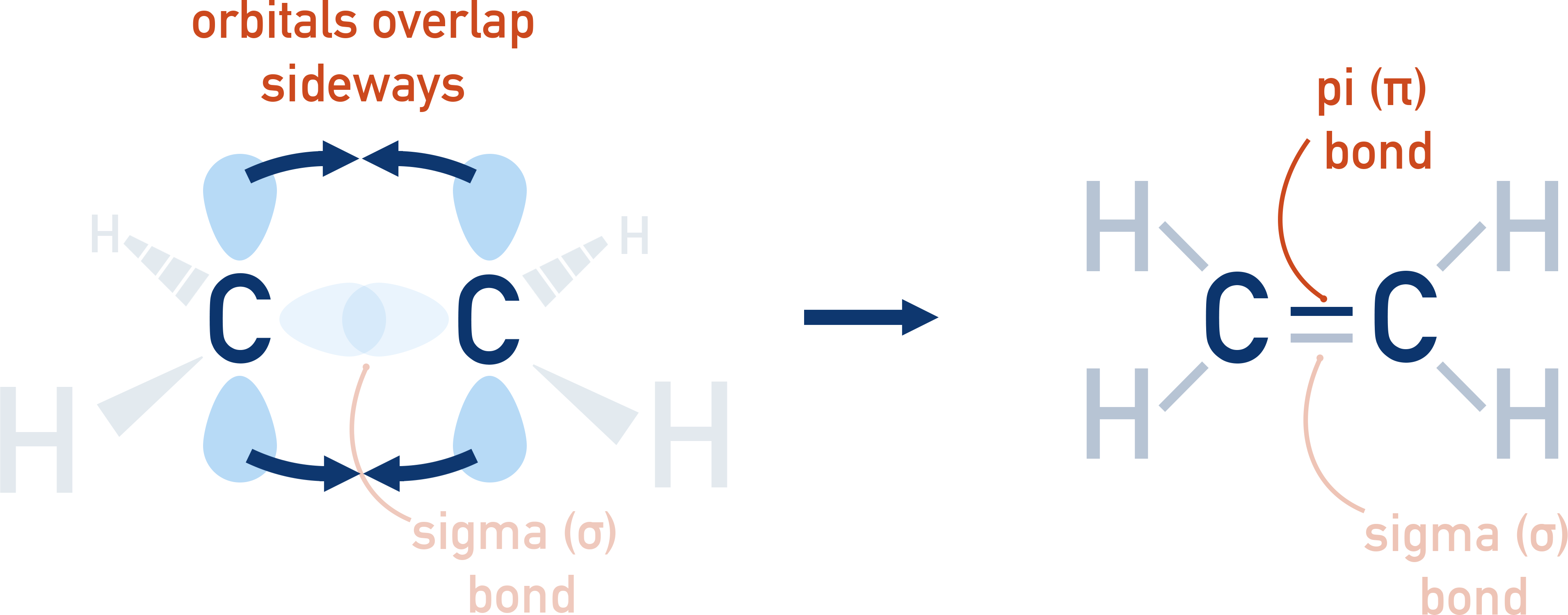
This diagram shows the σ-bond between the two carbon atoms in ethene and the π-bond formed by sideways p‑orbital overlap. The π-bond is more exposed and weaker, helping explain alkene reactivity. Source
π-bond: A bond formed by sideways overlap of adjacent p-orbitals, producing electron density above and below the plane of bonded atoms.
Because this π-bond is relatively easy to break, alkenes undergo addition reactions, where reactants add across the double bond to form a saturated product. These reactions allow the C=C bond to convert into two new σ-bonds, which are energetically more stable. As a result, addition reactions are generally favourable and occur under comparatively mild conditions.
Hydrogenation: Addition of Hydrogen (H₂)
Hydrogenation reduces an alkene to an alkane through addition of hydrogen across the double bond. The specification emphasises the use of H₂ with a nickel catalyst, which provides a surface for adsorption and activation of both reactants.
Key Features of Hydrogenation
Converts alkenes → alkanes
Requires H₂ and a nickel catalyst
Takes place under raised temperature (commonly around 150 °C)
Once hydrogen atoms add across the double bond, the product contains only C–C and C–H σ-bonds, resulting in a fully saturated molecule.
Halogenation: Addition of Halogens (X₂)
Halogenation occurs when a halogen molecule, typically Br₂ or Cl₂, reacts with an alkene to form a dihaloalkane. This reaction is also used as a qualitative analytical test for unsaturation because the characteristic colour of the halogen solution disappears.
Steps in Halogenation
Electrophilic attack by the halogen molecule on the electron-rich C=C bond
Formation of a dihaloalkane as two halogen atoms add across the double bond
Decolourisation of bromine water when testing for unsaturation
Electrophile: An electron-pair acceptor.
Following halogenation, the product contains two new C–X σ-bonds in place of the π-bond.
Hydrohalogenation: Addition of Hydrogen Halides (HX)
Hydrogen halides such as HCl, HBr, and HI add across the C=C bond to form haloalkanes. The general reaction is fast and does not require a catalyst, though conditions may vary depending on the halide.
Hydrohalogenation is important for producing a wide range of haloalkanes used in organic synthesis. The orientation of addition can depend on carbocation stability, but the syllabus requirement for this subsubtopic focuses on recognising the formation of haloalkanes rather than applying Markownikoff's rule.
Features of Hydrohalogenation
Reacts alkenes + HX → haloalkanes
Occurs quickly due to high electron density of C=C
Proceeds via addition across the double bond forming two new σ-bonds
Hydration: Addition of Steam (H₂O)
Hydration of alkenes produces alcohols, a process of major industrial importance, especially for manufacturing ethanol. The specification highlights the use of steam with a phosphoric acid catalyst (H₃PO₄).
Conditions Required
Steam (gaseous water)
H₃PO₄ catalyst (commonly on a solid support)
High temperature and pressure in industrial settings
Catalyst: A substance that increases reaction rate without being consumed, providing an alternative reaction pathway with lower activation energy.
Hydration leads to formation of an alcohol in which the OH group attaches to one of the carbons originally part of the C=C bond.
Alkenes react with steam in the presence of a phosphoric acid (H₃PO₄) catalyst at high temperature and pressure to form alcohols.
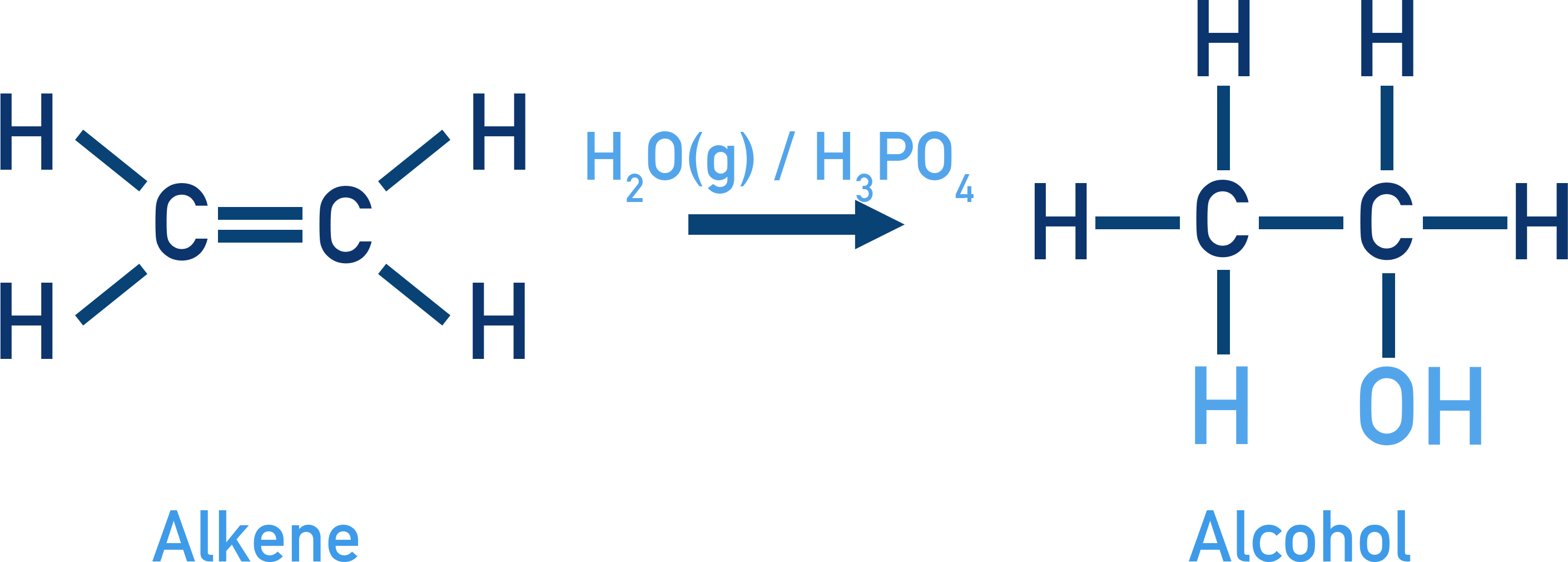
This diagram shows ethene reacting with steam in the presence of phosphoric acid, forming ethanol. The addition occurs across the C=C bond under industrial conditions. Source
Why Addition Reactions Occur Readily
The specification emphasises the lower enthalpy of the π-bond compared with a σ-bond. Because breaking the π-bond requires relatively little energy, the C=C bond is susceptible to attack by electrophiles and other reactants. The products of addition reactions contain stronger σ-bonds, which contributes to the overall thermodynamic favourability.
Normal alkanes do not undergo these reactions without extreme conditions because they lack the exposed π-bond. This contrast highlights the central role of π-bond weakness in defining the chemical behaviour of alkenes.
Summary of Key Addition Reactions Required by the Specification
Hydrogenation
Reagent: H₂
Catalyst: Ni
Product: Alkane
Key idea: Conversion of C=C to two σ-bonds through hydrogen addition
Halogenation
Reagent: X₂ (e.g., Br₂, Cl₂)
Product: Dihaloalkane
Key idea: Rapid electrophilic addition; loss of halogen colour indicates unsaturation
Hydrohalogenation
Reagent: HX (e.g., HCl, HBr)
Product: Haloalkane
Key idea: Addition without catalyst, forming two new σ-bonds
Hydration
Reagents: Steam/H₂O(g)
Catalyst: H₃PO₄
Product: Alcohol
Key idea: Industrial synthesis of alcohols via addition across the C=C bond
Alkenes undergo a range of addition reactions in which the π-bond is broken and new σ-bonds form, such as hydrogenation, halogenation and hydration.
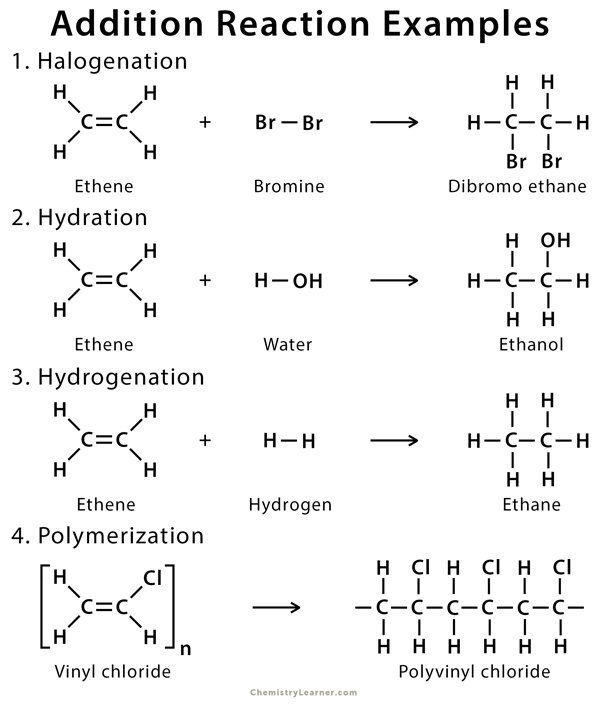
This diagram summarises addition reactions of ethene, including halogenation, hydration, and hydrogenation. Each involves breaking the π-bond to form new σ-bonds. The polymerisation example includes extra detail beyond this syllabus. Source
FAQ
The π-bond creates regions of high electron density above and below the carbon–carbon bond. Electrophiles are attracted to these regions because they seek electron-rich sites.
This means the initial step in most alkene addition reactions involves the electrophile approaching the π-bond rather than the σ-framework of the molecule.
The orientation of attack influences the intermediate formed, especially in stepwise mechanisms.
Reactions such as hydrohalogenation often proceed via carbocations because HX can polarise easily, allowing heterolytic bond breaking and stepwise addition.
In contrast, halogenation tends to form a cyclic halonium ion intermediate instead of a carbocation due to the ability of halogens to bridge the two carbons.
The pathway depends on the nature of the electrophile and how charge can best be stabilised.
Stability is influenced by:
Degree of alkyl substitution (tertiary > secondary > primary)
Ability to spread positive charge through hyperconjugation
Whether a bridged or open intermediate minimises energy
More stable intermediates lower activation energy, making a reaction pathway favoured.
Industrially, hydration must produce alcohols at high yield and rate, requiring elevated temperature and pressure to optimise equilibrium and reaction speed.
In laboratory settings, conditions are milder because the goal is demonstration rather than maximising throughput.
Industry also uses solid phosphoric acid catalysts for durability and continuous-flow processing.
Hydrogenation occurs on a metal catalyst surface where bonds in H2 weaken as the molecule adsorbs, allowing stepwise transfer of hydrogen atoms to the alkene.
Halogenation takes place entirely in solution and involves electrophilic attack by a polarised halogen molecule, forming a cyclic halonium ion before addition completes.
These mechanistic differences reflect the contrasting properties of H2 and halogens as reagents.
Practice Questions
Explain why alkenes are more reactive than alkanes.
(2 marks)
Alkenes contain a π-bond that has lower bond enthalpy than a σ-bond. (1)
The π-bond is more exposed/easily broken, making the C=C bond susceptible to attack by electrophiles. (1)
Ethene undergoes several addition reactions. Describe the conditions and products for:
a) Hydrogenation
b) Hydration
c) Reaction with bromine
Explain why all of these reactions involve breaking the same type of bond in ethene.
(5 marks)
a) Hydrogenation
Ethene reacts with hydrogen in the presence of a nickel catalyst. (1)
Forms ethane (an alkane). (1)
b) Hydration
Ethene reacts with steam using a phosphoric acid catalyst (H3PO4). (1)
Forms ethanol. (1)
c) Reaction with bromine
Bromine adds across the C=C double bond. (1)
Forms 1,2-dibromoethane. (1)
Explanation
All reactions involve breaking the π-bond in the C=C double bond because it is weaker and easier to break than a σ-bond. (1)
(Any 5 marks total; if more points offered, award best 5)

