OCR Specification focus:
‘Alkenes contain a C=C with one π-bond from sideways p-orbital overlap above/below the plane and one σ-bond; π-bonds restrict rotation; explain trigonal planar geometry at C=C.’
Alkenes possess characteristic bonding features that determine their geometry and reactivity, with σ-bonds, π-bonds, and restricted rotation forming the core ideas needed for understanding this subsubtopic.
The Nature of σ-Bonds in Alkenes
Alkenes are hydrocarbons containing at least one carbon–carbon double bond (C=C), which is composed of two distinct bonding interactions: a σ-bond and a π-bond. The σ-bond forms the foundational single-bond framework of all organic molecules and is the first component of every multiple bond.
A σ-bond arises from direct, end-to-end overlap of orbitals, typically between two sp² hybrid orbitals on adjacent carbon atoms in an alkene. This head-on overlap produces a region of high electron density located directly between the nuclei.
σ-bond: A covalent bond formed by the direct, end-to-end overlap of atomic orbitals, concentrating electron density along the internuclear axis.
Because electron density in σ-bonds is symmetrical around the bond axis, these bonds allow free rotation in alkanes. However, in alkenes this rotational freedom is lost due to the presence of the π-bond. The σ-bond remains essential for maintaining the strong, stable connection between the carbon atoms and supports the planar shape at the C=C region.
Formation and Characteristics of π-Bonds
The second component of the C=C double bond is the π-bond, which plays a crucial role in restricting molecular movement and influencing chemical behaviour. A π-bond forms only after the σ-bond is established, using the remaining unhybridised p-orbitals on each carbon atom.
The p-orbitals overlap sideways, creating electron density above and below the plane of the σ-bond.

Diagram of ethene showing the π-bond formed by sideways overlap of adjacent p-orbitals. The shaded lobes above and below the C=C bond represent regions of π-electron density. This visual highlights how the π-bond sits in addition to the σ-bond along the internuclear axis and helps explain its greater reactivity. Source
This distribution of electron density produces a weaker, more exposed bond that is more chemically reactive.
π-bond: A covalent bond formed by sideways overlap of unhybridised p-orbitals, producing electron density above and below the plane of the bonding atoms.
The presence of a π-bond introduces a rigid structure around the double bond, directly linking to the restricted rotation central to this subsubtopic.
The dual-layer electron density in the π-bond prevents one carbon from rotating independently around the bond axis without breaking the π-bond. As breaking a π-bond requires significant energy input, rotation is effectively prevented under normal conditions.
Restricted Rotation at the C=C Bond
Restricted rotation is a defining structural feature of alkenes. Because the π-bond occupies a fixed position relative to the σ-bond, the two carbon atoms in the double bond cannot twist around each other freely. Rotation would disrupt the overlapping p-orbitals and destroy the π-bond.
This leads to important spatial consequences: the groups attached to each carbon are locked in position, giving rise to stereoisomerism in many alkenes. Although stereochemistry is handled elsewhere in the specification, it is important here to understand that restricted rotation is the root cause of E/Z arrangements.
Key reasons why rotation is restricted:
The π-bond occupies a fixed region of electron density above and below the plane.
Sideways overlap of p-orbitals can only be maintained if the orbitals remain parallel.
Twisting the molecule breaks this alignment and therefore breaks the π-bond.
Breaking a π-bond requires substantial energy, so alkenes retain fixed positions around C=C in normal conditions.
As breaking a π-bond requires significant energy input, rotation is effectively prevented under normal conditions.

Orbital diagrams illustrating how rotation about a carbon–carbon bond affects orbital overlap. The left-hand part shows that a pure σ-bond can rotate without changing overlap, whereas the right-hand part shows that rotation in a σ + π system breaks the p-orbital overlap and therefore the π-bond. The figure includes extra conceptual detail but supports the idea that the π-bond locks the C=C and restricts rotation. Source
Geometry Around the C=C Bond
The OCR specification requires explanation of the trigonal planar geometry around each carbon in an alkene’s double bond. Each carbon in the C=C uses three sp² hybrid orbitals, arranged around the atom with approximate bond angles of 120°.
Each carbon in the C=C uses three sp² hybrid orbitals, arranged around the atom with approximate bond angles of 120°.
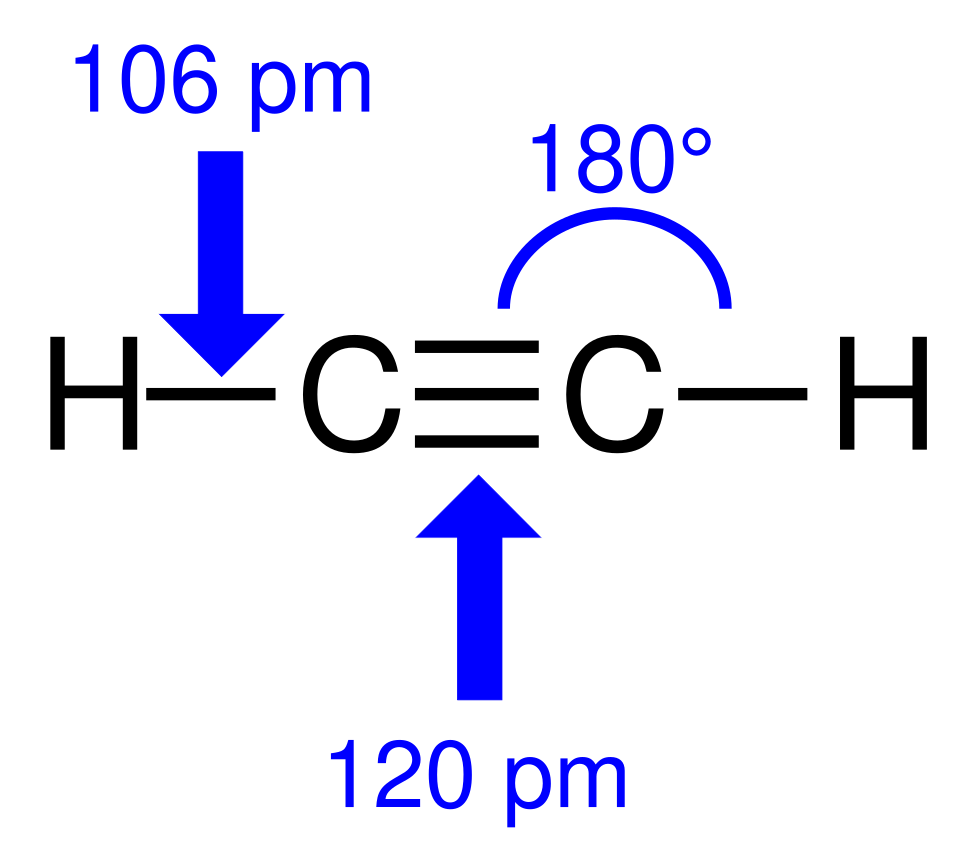
Lewis structure of ethene with labelled bond angles and bond lengths. The diagram shows each carbon as trigonal planar, with H–C–H and H–C=C angles close to 120°, consistent with sp² hybridisation. The labelled bond lengths provide useful additional structural detail beyond the OCR specification. Source
This geometry arises from electron pair repulsion between bonding regions. With three bonding regions and no lone pairs at the double-bonded carbons, repulsion is evenly distributed, producing a flat trigonal planar arrangement.
Trigonal planar geometry: A molecular shape in which three bonding regions arrange themselves around a central atom at approximately 120° in a single plane.
Alkenes therefore have a planar section around the C=C, where all atoms directly bonded to the double-bonded carbons lie in the same plane. This planar arrangement is essential for maintaining p-orbital overlap, further reinforcing restricted rotation.
Significance of σ- and π-Bonds for Alkene Behaviour
Understanding the combination of σ-bonds and π-bonds provides insight into the structural and reactive properties of alkenes:
σ-bond: Strong, localised electron density; provides the core carbon–carbon framework.
π-bond: Weaker, more exposed electron density; responsible for increased reactivity.
Restricted rotation: Prevents twisting around the double bond, fixing atom positions.
Trigonal planar shape: Ensures proper p-orbital alignment and consistent geometry across alkenes.
FAQ
A pi bond is weaker than a sigma bond because its sideways overlap is less effective and more exposed to attack by reagents.
This matters in alkenes because:
The weaker pi bond is the first part of the double bond to react.
Electrophiles are attracted to the high electron density in the pi region.
Breaking the pi bond while keeping the sigma bond intact allows additions to occur.
Pi-bond formation relies on continuous sideways overlap between two unhybridised p-orbitals.
If the orbitals are not parallel:
Their overlap is reduced or lost entirely.
The pi bond collapses because electron density cannot be sustained above and below the plane.
Rotation around the double bond becomes impossible without breaking the pi system.
Each carbon in an alkene needs one unhybridised p-orbital for the pi bond, so only three orbitals are hybridised to form sp2.
This results in:
Three equivalent sp2 orbitals for sigma bonding.
A trigonal planar arrangement allowing optimum overlap.
A remaining p-orbital positioned correctly for pi-bond formation.
Because electron density is located above and below the plane of the molecule, it is more exposed than in a sigma bond.
This leads to:
Increased susceptibility to electrophilic attack.
Greater polarisation effects when reagents approach the double bond.
Reactions that target the pi region without initially disturbing the sigma framework.
Restricted rotation fixes substituents in specific orientations, affecting an alkene’s shape and interactions.
Consequences include:
Differences in boiling points or melting points between E and Z isomers.
Variations in polarity depending on substituent arrangement.
Distinct spatial arrangements that influence reactivity and intermolecular forces.
Practice Questions
Explain why rotation around the carbon–carbon double bond in an alkene does not occur under normal conditions.
(2 marks)
Award one mark for each correct point:
Rotation would break the pi bond because sideways overlap of p-orbitals cannot be maintained.
Breaking the pi bond requires significant energy, so rotation does not occur under normal conditions.
Ethene contains both a sigma bond and a pi bond between the two carbon atoms.
Describe how each of these bonds is formed and explain how they determine both the geometry around the double bond and the restricted rotation observed in alkenes.
(5 marks)
Award marks for the following points (maximum 5 marks):
Sigma bond formed by direct end-to-end overlap of orbitals between the carbon atoms.
Pi bond formed by sideways overlap of unhybridised p-orbitals above and below the plane of the sigma bond.
Each carbon in the C=C uses three sp2 hybrid orbitals, giving trigonal planar geometry.
Bond angles around each carbon are approximately 120 degrees due to electron pair repulsion.
Restricted rotation occurs because rotating the carbons would break the pi-bond overlap, which requires significant energy.

