OCR Specification focus:
‘Define nucleophile as an electron pair donor; show nucleophilic substitution mechanism for primary haloalkanes with aqueous alkali using curly arrows and relevant dipoles.’
Nucleophilic substitution is a fundamental transformation of haloalkanes, allowing predictable changes to molecular structure through well-defined mechanisms involving electron-pair donation and bond reorganisation.
Nucleophiles: Nature and Reactivity
Nucleophiles play a central role in substitution reactions because they provide an electron pair to form a new covalent bond. They commonly bear lone pairs and may be neutral or negatively charged.
Nucleophile: A species that donates an electron pair to an electron-deficient atom to form a new covalent bond.
Nucleophiles target electrophilic centres, and in haloalkanes the carbon attached to the halogen is strongly polarised. The electronegative halogen withdraws electron density, creating a δ+ carbon susceptible to attack. Haloalkanes such as chloroethane, bromoethane, and iodoethane therefore undergo substitution when exposed to suitable nucleophiles.
Factors Affecting Nucleophilic Strength
Nucleophilic strength depends on:
Charge – negatively charged species (e.g. OH⁻, CN⁻) are generally stronger nucleophiles than neutral molecules.
Electronegativity – nucleophiles with lower electronegativity donate electron density more readily.
Solvent – polar protic solvents can hinder nucleophiles through hydrogen bonding, while polar aprotic solvents enhance nucleophilicity for many anions.
Steric hindrance – bulky nucleophiles attack electrophilic centres less effectively.
These factors influence rate, but under OCR requirements, emphasis remains on substitution involving aqueous alkali and primary haloalkanes.
Nucleophilic Substitution of Primary Haloalkanes
Primary haloalkanes undergo nucleophilic substitution through the S(_N)2 mechanism. The specification requires students to show the substitution mechanism using curly arrows and apply correct dipoles.
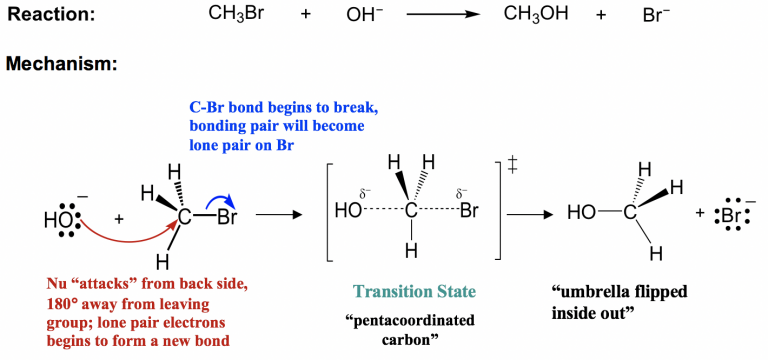
This image illustrates concerted SN2 attack with curly arrows showing electron movement, a pentacoordinate transition state, and formation of methanol and bromide. Source
Polarity of the C–X Bond
In haloalkanes, the halogen (X) is more electronegative than carbon. This produces:
Cδ+, an electrophilic carbon centre
Xδ−, capable of leaving as a halide ion
This polarisation is essential because the carbon atom has an accessible δ+ site for nucleophilic attack.
The S(_N)2 Mechanism: Key Features
The mechanism for primary haloalkanes with aqueous alkali proceeds in one concerted step, meaning bond breaking and bond forming occur simultaneously.
The reaction involves:
A nucleophile such as OH⁻ from aqueous alkali
A primary haloalkane (e.g. bromoethane)
Formation of an alcohol and release of a halide ion
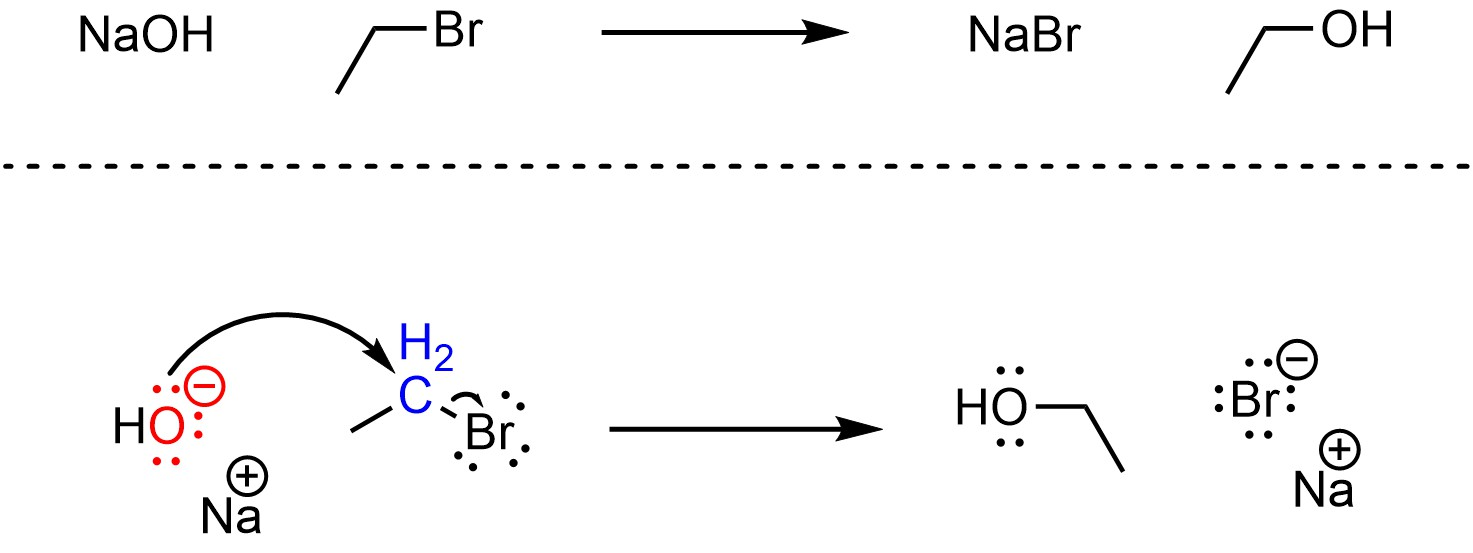
This scheme shows hydroxide attacking bromoethane in one step to produce ethanol and bromide, emphasising simultaneous bond-making and bond-breaking. Source
Important features of the S(_N)2 process include:
Back-side attack: the nucleophile approaches the carbon opposite the C–X bond.
Transition state formation: carbon simultaneously bonds partially to the nucleophile and halogen.
Inversion of configuration at carbon, though OCR does not require stereochemical drawings.
Step-by-Step Description of the Required Mechanism
1. Nucleophilic Approach
The OH⁻ nucleophile approaches the δ+ carbon from the opposite side of the halogen. This direction is essential because it minimises repulsion between the nucleophile and the electron-rich halogen.
2. Curly Arrow Representation
A curly arrow originates from the lone pair on the nucleophile and points towards the carbon atom. A second curly arrow emerges from the C–X bond to the halogen, representing bond cleavage.
3. Formation of the Transition State
As the nucleophile forms a new bond with carbon, the C–X bond weakens. In the transition state:
Both nucleophile and halogen are partially bonded to carbon.
The carbon adopts a geometry with five partial bonds momentarily.
Example identity of species is not required beyond primary haloalkanes and aqueous alkali.
A normal sentence separates blocks of structured content to maintain clarity and flow.
4. Product Formation
The halogen leaves as X⁻ (a halide ion), while the nucleophile fully bonds to carbon, forming an alcohol. The reaction therefore converts a haloalkane into an alcohol, aligning with fundamental synthetic pathways introduced in Module 4.
Why Primary Haloalkanes Use S(_N)2
OCR expects students to understand that primary haloalkanes favour the S(_N)2 mechanism due to minimal steric hindrance. Secondary haloalkanes may undergo either S(_N)1 or S(_N)2, and tertiary haloalkanes typically undergo S(_N)1, but these comparisons fall outside the direct specification for this subsubtopic.
Primary haloalkanes’ relatively open structure allows the nucleophile to approach directly along the required trajectory for back-side attack. The mechanism therefore remains both predictable and high-yielding under aqueous alkali conditions.
Relevance of Dipoles in Mechanistic Diagrams
When drawing the mechanism, OCR requires:
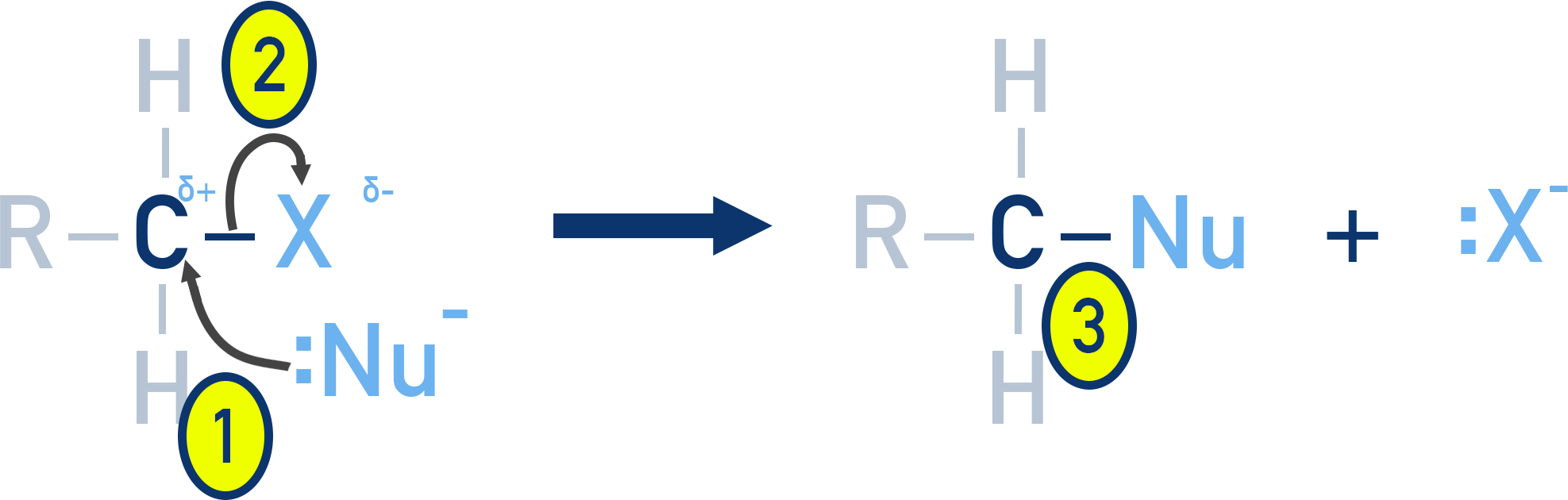
This diagram highlights dipole placement and curly arrow conventions in SN2 mechanisms, illustrating nucleophilic attack and departure of the leaving group. Source
δ+ clearly placed on carbon
δ− on the halogen
Curly arrows accurately directed: lone pair → carbon; C–X bond → halogen
Correct placement of dipoles is not optional; it demonstrates mechanistic understanding rather than memorised arrows.
Key Mechanistic Bullet Points for OCR Practice
Nucleophile: electron pair donor attacking Cδ+
Primary haloalkane: undergoes S(_N)2 substitution
Aqueous alkali: common nucleophile source (OH⁻)
Curly arrows: from nucleophile to carbon; from C–X bond to halogen
Products: alcohol + halide ion
Back-side attack: essential for mechanism
Polarisation: C–X bond dipole drives reactivity
FAQ
The weaker the carbon–halogen bond, the more readily the leaving group departs, increasing the rate of SN2 substitution.
Iodoalkanes react fastest because the C–I bond is weakest, while fluoroalkanes are effectively unreactive due to the strong C–F bond.
This trend affects reaction rate but does not alter the single-step SN2 mechanism used by primary haloalkanes.
Back-side attack aligns the nucleophile with the antibonding orbital of the C–X bond, allowing simultaneous bond formation and bond breaking.
Front-side attack is energetically unfavourable because the nucleophile would encounter electron repulsion from the halogen and filled orbitals on carbon.
A good leaving group must:
Form a stable anion after departure
Have a low basicity
Allow effective overlap between the carbon’s bonding and antibonding orbitals during the transition state
Halide ions meet these conditions, with iodide being the best leaving group in typical SN2 chemistry.
Polar aprotic solvents, such as propanone or dimethyl sulfoxide, enhance nucleophilicity by preventing hydrogen bonding to anions.
In contrast, polar protic solvents stabilise nucleophiles through solvation, slowing SN2 reactions.
Although OCR does not require practical solvent selection, understanding these effects helps explain variability in reaction rates.
During the SN2 step, carbon temporarily interacts with both the nucleophile and the leaving group, giving rise to two partial bonds.
This reflects the concerted nature of the mechanism, where no intermediates form.
The geometry resembles a flattened, trigonal bipyramidal arrangement, consistent with simultaneous electron-pair movement.
Practice Questions
Chloroethane undergoes nucleophilic substitution when reacted with aqueous hydroxide ions.
(a) Define the term nucleophile.
(b) State the role of the hydroxide ion in this reaction.
(2 marks)
(a) Nucleophile definition:
Species that donates an electron pair to form a covalent bond. (1 mark)
(b) Role of hydroxide ion:
Acts as the nucleophile / electron pair donor in the substitution reaction. (1 mark)
Bromoethane reacts with aqueous hydroxide ions in a single-step SN2 mechanism.
(a) Explain why the carbon atom in bromoethane is susceptible to attack by nucleophiles.
(b) Describe, using curly arrows, the key steps in the SN2 mechanism for this reaction.
(c) Explain why primary haloalkanes such as bromoethane favour the SN2 mechanism rather than the SN1 mechanism.
(5 marks)
(a) Susceptibility of carbon to nucleophilic attack:
The C–Br bond is polar. (1 mark)
Carbon is δ+ because bromine is more electronegative, making it electrophilic. (1 mark)
(b) Key steps in SN2 mechanism (curly arrow description):
Curly arrow from lone pair on OH⁻ to the δ+ carbon. (1 mark)
Curly arrow from the C–Br bond to the bromine atom, showing departure as Br⁻. (1 mark)
(c) Primary haloalkanes favouring SN2:
Minimal steric hindrance around the carbon allows back-side attack. (1 mark)
(Any one correct point required for the final mark; both acceptable but only 1 mark available.)

