OCR Specification focus:
‘Explain UV photolysis of CFCs to halogen radicals and the catalysed breakdown of ozone; write equations for radical formation and O₃ destruction; appreciate evidence and policy responses.’
Modern environmental chemistry places strong emphasis on understanding how chlorofluorocarbons (CFCs) form radicals in the upper atmosphere and how these radicals catalyse the destruction of ozone, affecting global policy decisions.
Chlorofluorocarbons (CFCs): Structure and Properties
CFCs are halogenoalkanes containing only chlorine, fluorine, and carbon. Their stability, non-toxicity, and non-flammability once made them widely used in aerosols, refrigerants, and foam manufacturing. This chemical stability is crucial: it allows CFCs to remain intact long enough to reach the stratosphere, where UV radiation becomes sufficiently energetic to break bonds.
Radical: A highly reactive species containing an unpaired electron.
Although stable in the troposphere, CFCs undergo photochemical reactions under intense UV light, generating radicals capable of participating in catalytic ozone destruction cycles.
UV Photolysis of CFCs
Strong UV radiation in the stratosphere provides enough energy to break the carbon–chlorine bond in CFCs. This process is known as photolysis, the homolytic cleavage of a covalent bond by UV radiation.
Photolysis of CFCs (CCl₃F) = CCl₃F → •CCl₂F + •Cl
CCl₃F = Trichlorofluoromethane
•Cl = Chlorine radical
The chlorine radical formed is extremely reactive because its unpaired electron readily participates in chemical reactions. These radicals initiate a chain process that destroys atmospheric ozone.
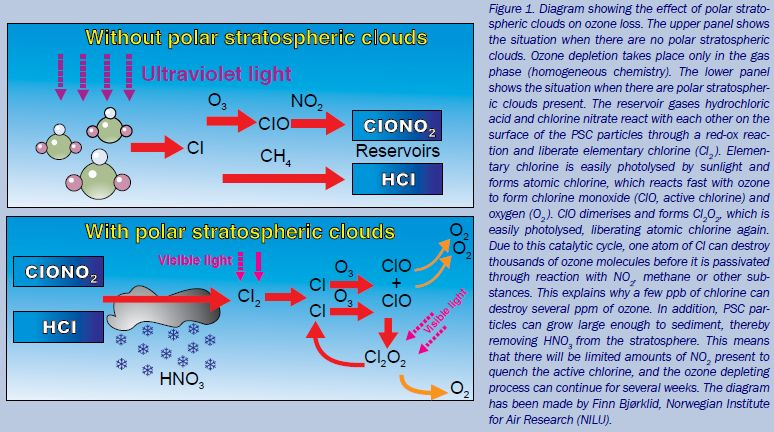
This diagram illustrates how ultraviolet radiation breaks a CFC molecule (CFCl₃), releasing a chlorine radical. The chlorine radical then follows a two-step catalytic cycle, converting ozone into oxygen while the chlorine atom is regenerated. The image also notes that a single chlorine atom can destroy many ozone molecules, which slightly extends beyond the specification but reinforces the catalytic nature of the process. Source
Once formed, chlorine radicals experience little removal because they are regenerated during the catalytic cycle. This property contributes significantly to their long atmospheric lifetime and environmental impact.
The Ozone Layer and Its Vulnerability
The ozone layer absorbs harmful UV-B and UV-C radiation, protecting biological systems from DNA damage, reduced crop yield, and increased skin cancer risk.
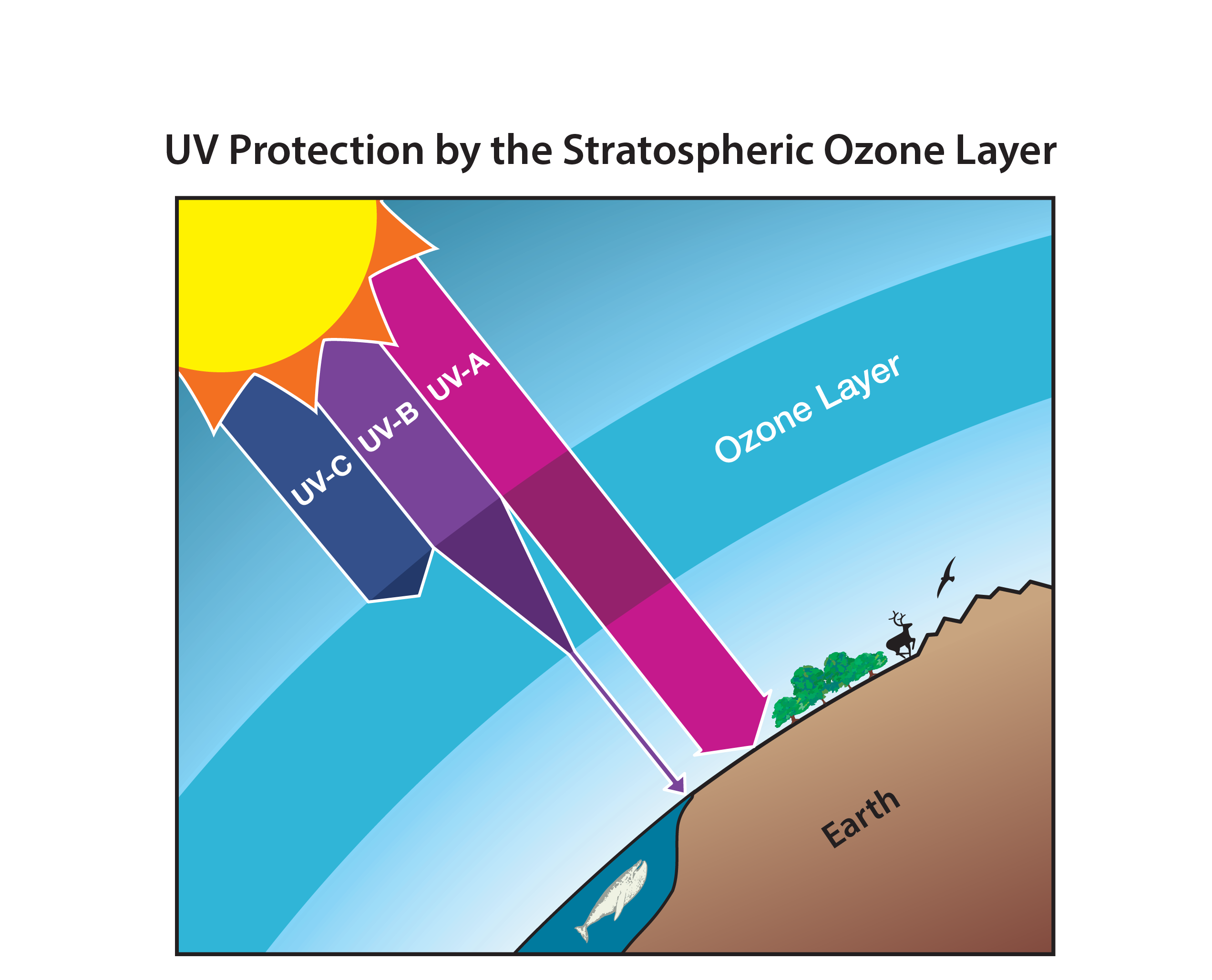
This illustration shows the ozone layer in the stratosphere and three coloured beams representing UV-C, UV-B and UV-A radiation from the Sun. It highlights that UV-C and most UV-B are absorbed by ozone, while some UV-B and most UV-A reach Earth’s surface. The diagram includes extra detail on wavelength ranges and biological effects, which goes slightly beyond the A-Level syllabus but reinforces why ozone depletion is a major environmental concern. Source
Ozone is continuously formed and broken down naturally, but radical species from CFCs disrupt this balance.
Ozone (O₃): A triatomic form of oxygen that strongly absorbs UV radiation.
When radicals enter this equilibrium system, they catalyse the removal of ozone far faster than natural processes can replace it, lowering ozone concentration in the stratosphere.
Radical-Catalysed Ozone Destruction Mechanism
Chlorine radicals react with ozone in a catalytic cycle. Because the radical is regenerated at the end, each chlorine radical can destroy thousands of ozone molecules.
The catalytic destruction process involves the following steps:
Step 1: Reaction of Chlorine Radical with Ozone
• Cl radical reacts with an ozone molecule, forming chlorine monoxide (ClO•) and molecular oxygen.
• This step removes one ozone molecule from the atmosphere.
Ozone Decomposition Step 1 = •Cl + O₃ → ClO• + O₂
•Cl = Chlorine radical
O₃ = Ozone
ClO• = Chlorine monoxide radical
The reaction forms a new radical species, ClO•, which remains active in the catalytic cycle.
Step 2: Regeneration of the Chlorine Radical
• ClO• reacts with atomic oxygen (O), forming O₂ and regenerating the chlorine radical.
• The regenerated radical continues to destroy further ozone molecules.
Ozone Decomposition Step 2 = ClO• + O → •Cl + O₂
ClO• = Chlorine monoxide radical
O = Atomic oxygen
•Cl = Regenerated chlorine radical
A normal sentence must appear here before any further structural information is introduced.
Overall, the radical remains available to continue reacting, making this a highly efficient catalytic cycle with significant environmental consequences.
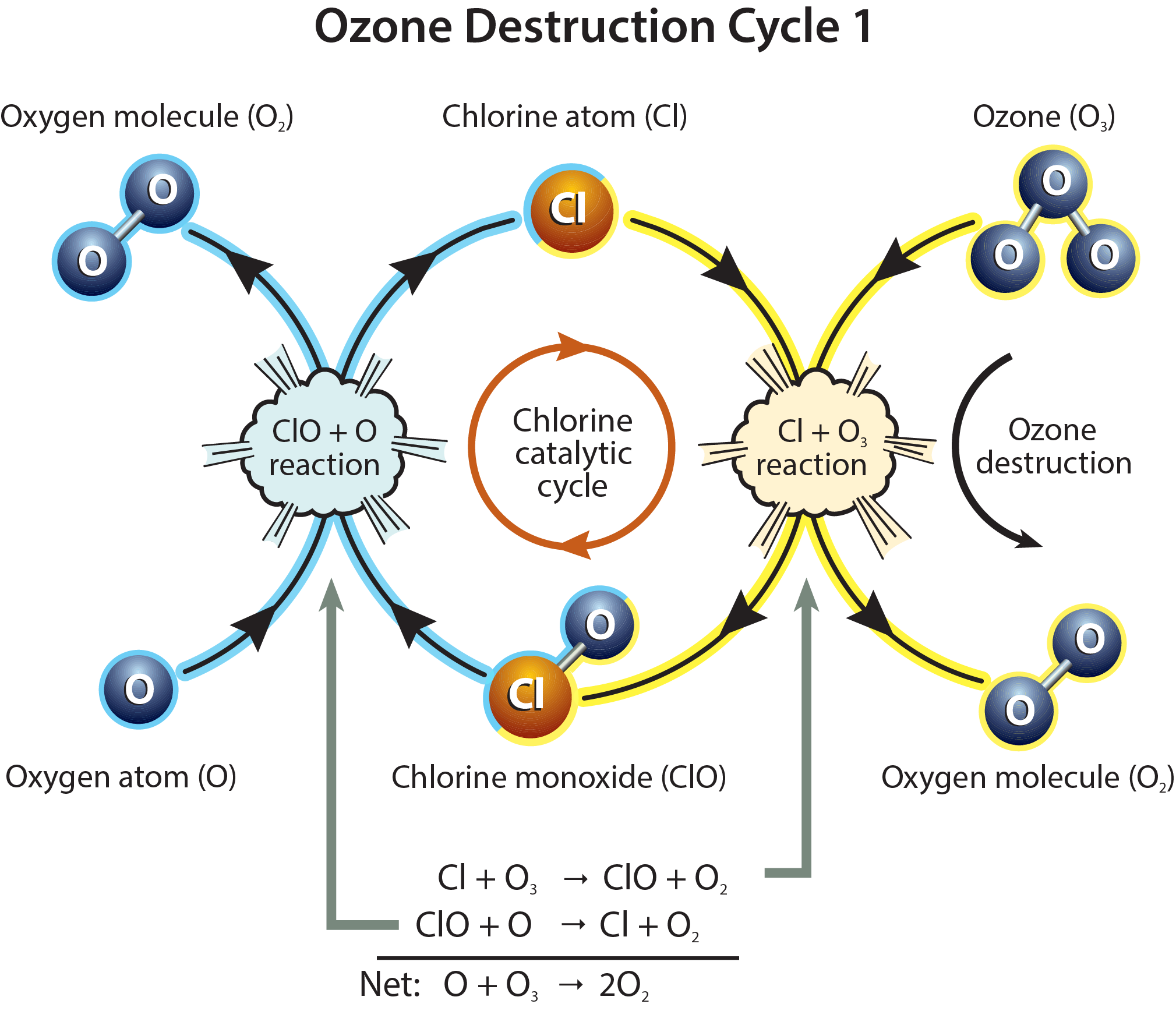
This diagram presents Ozone Destruction Cycle 1, in which a chlorine radical reacts with ozone to form chlorine monoxide and oxygen, then chlorine monoxide reacts with atomic oxygen to regenerate the chlorine radical. Arrows show the continuous loop of reactions, emphasising that chlorine is a catalyst that is re-formed at the end of each cycle. The figure also includes the net equation O + O₃ → 2O₂ and labels for the species involved, which add detail consistent with, but slightly more explicit than, the A-Level specification. Source
FAQ
CFCs with more carbon–chlorine bonds are generally more susceptible to UV photolysis because the C–Cl bond is weaker than the C–F bond, requiring less energy to break.
This means CFCs containing several chlorine atoms tend to release radicals more readily once they reach the stratosphere, increasing their impact on ozone depletion.
Chlorine radicals participate in cycles that regenerate them, so they are not consumed in the destruction of ozone.
Additionally, the stratosphere contains relatively few substances capable of reacting with chlorine radicals to remove them permanently, leading to long radical lifetimes.
Chlorine radicals can be temporarily or permanently removed through formation of reservoir species such as hydrochloric acid and chlorine nitrate.
These reactions typically occur on polar stratospheric cloud surfaces, especially during polar winters, reducing the concentration of active radicals.
Polar stratospheric clouds provide surfaces for heterogeneous reactions that convert reservoir species (such as HCl and ClONO2) into photolabile forms like Cl2.
When sunlight returns, Cl2 undergoes photolysis to release large amounts of chlorine radicals, accelerating ozone destruction.
Antarctica experiences exceptionally low winter temperatures, enabling widespread polar stratospheric cloud formation.
The prolonged darkness allows reservoir species to accumulate on cloud surfaces, and the sudden return of sunlight in spring triggers rapid radical release, creating the pronounced seasonal ozone hole.
Practice Questions
State what is meant by the term radical and explain why chlorine radicals formed from CFCs are particularly significant in the stratosphere.
(2 marks)
1 mark: Radical defined as a species with an unpaired electron.
1 mark: Chlorine radicals are significant because they catalyse ozone decomposition / are regenerated and can destroy many ozone molecules.
CFCs undergo photolysis in the stratosphere, releasing chlorine radicals that take part in catalytic ozone destruction.
(a) Write the two equations that show how chlorine radicals catalyse the breakdown of ozone.
(b) Explain why this process is considered catalytic and discuss one piece of scientific evidence that led to international action to limit CFC use.
(5 marks)
Part (a):
1 mark: Cl + O3 → ClO + O2
1 mark: ClO + O → Cl + O2
Part (b):
1 mark: Explanation that chlorine radicals are regenerated at the end of the cycle.
1 mark: Therefore a single radical can destroy many ozone molecules (acts as a catalyst).
1 mark: Evidence such as measurements of stratospheric ozone depletion over Antarctica / detection of chlorine compounds in the stratosphere linked to CFCs / NASA or satellite data showing progressive ozone thinning.

