OCR Specification focus:
‘Explain trend in hydrolysis rates of primary haloalkanes in terms of C–X bond enthalpies (C–F strongest to C–I weakest); link to experimental observations.’
Hydrolysis rates of primary haloalkanes vary significantly due to differences in carbon–halogen bond enthalpies, influencing how readily nucleophilic substitution occurs and determining observed experimental trends in reactivity.
Rates and Bond Enthalpy Trend
Understanding the Link Between Bond Enthalpy and Hydrolysis
The hydrolysis of haloalkanes is a nucleophilic substitution reaction in which a nucleophile, commonly aqueous hydroxide ions, attacks the electron-deficient carbon bonded to a halogen. The ease of hydrolysis depends strongly on the C–X bond enthalpy, a measure of the energy required to break the carbon–halogen bond. Lower bond enthalpy corresponds to weaker bonds that break more readily, leading to faster hydrolysis.
Bond Enthalpy: The energy required to break one mole of a specific covalent bond in the gaseous state.
Primary haloalkanes are the focus here because they undergo nucleophilic substitution through a single-step mechanism, making comparisons in bond enthalpy and rate behaviour particularly clear.
Comparative Bond Strengths in Haloalkanes
The trend in carbon–halogen bond enthalpies is a direct reflection of halogen atomic properties. Moving down Group 17, atoms increase in size and the bond formed with carbon lengthens and weakens. This produces the characteristic order:
C–F: strongest bond (highest bond enthalpy)
C–Cl: moderate strength
C–Br: weaker bond
C–I: weakest bond (lowest bond enthalpy)
This ranking directly determines the hydrolysis rate trend.
Because C–F has the highest bond enthalpy and C–I the lowest, breaking a C–F bond requires far more energy than breaking C–Cl, C–Br or C–I.
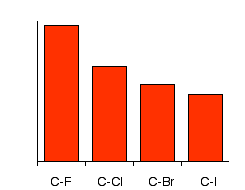
This chart compares C–X bond energies, showing that bond enthalpy decreases down the group from C–F to C–I. This explains why iodoalkanes hydrolyse fastest while fluoroalkanes remain largely unreactive under normal laboratory conditions. Source
Substitution Pathway and Breaking the C–X Bond
During hydrolysis with aqueous alkali, the rate-determining step is the breaking of the C–X bond as the nucleophile approaches. Because the nucleophile acts as an electron pair donor, it requires sufficient access to the positively polarised carbon.
Nucleophile: A species that donates an electron pair to form a new covalent bond.
Bond enthalpy therefore becomes a central factor: the weaker the bond, the less energy required for the nucleophile to displace the halide ion.
Experimental Observations Using Hydrolysis Tests
Experimentally, hydrolysis rate comparisons are often monitored using a mixture of ethanol, water, and silver nitrate. Silver ions react with the released halide ions to form insoluble silver halide precipitates. The time taken for the precipitate to appear correlates with hydrolysis rate.
1-iodoalkanes: fastest hydrolysis; yellow AgI precipitate appears quickly.
1-bromoalkanes: moderate rate; cream AgBr precipitate forms after a delay.
1-chloroalkanes: slow hydrolysis; white AgCl precipitate forms slowly.
1-fluoroalkanes: essentially no observable hydrolysis; no precipitate, because C–F bonds are extremely strong.
The faster the coloured silver halide precipitate appears, the faster the rate of hydrolysis of that haloalkane.
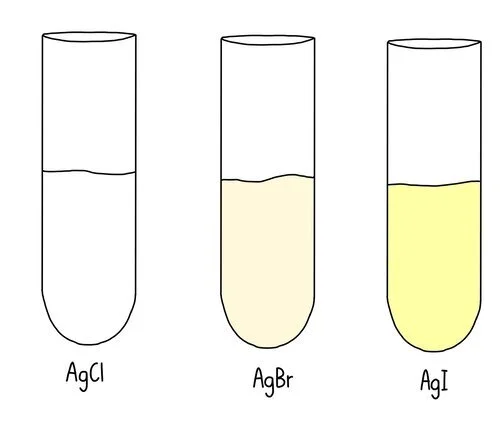
This diagram displays the characteristic silver halide precipitates—white AgCl, cream AgBr, and yellow AgI—formed during hydrolysis tests. Their appearance time provides experimental evidence for the trend in hydrolysis rates down Group 17. Source
Why C–F Is an Exceptionally Strong Bond
The C–F bond has a very high bond enthalpy because:
Fluorine’s small atomic radius allows highly effective orbital overlap with carbon.
The bond is short and highly polar, increasing electrostatic attraction.
The partial positive charge on carbon is stabilised strongly, making nucleophilic attack difficult.
These characteristics mean primary fluoroalkanes undergo hydrolysis extremely slowly under normal laboratory conditions.
Down-Group Trends Explained
The decreasing hydrolysis rate from iodo- to fluoro-compounds can be attributed to:
Increasing atomic radius down the group, causing poorer overlap with carbon orbitals.
Decreasing bond enthalpy, so less energy is required to break the bond.
Greater ease of leaving group departure, with iodide being the best leaving group due to its larger size and charge dispersal.
This explains why the C–I bond, being weakest, breaks most readily, enabling very fast nucleophilic substitution.
The Role of the Leaving Group
A leaving group is the species displaced from the substrate during substitution, taking with it the bonding electron pair. For haloalkanes, the halide ion is the leaving group.
Leaving Group: An atom or group that departs with an electron pair during bond breaking in a substitution reaction.
After understanding leaving groups, it becomes clear why bond enthalpy is the dominant factor in determining hydrolysis rate within primary haloalkanes.
Bond Enthalpy as the Key Determinant of Reactivity
Although factors such as polarity can influence some reactions, for primary haloalkanes undergoing hydrolysis the decisive factor is bond enthalpy, not bond polarity. Even though the C–Cl bond is more polar than the C–I bond, the much higher bond enthalpy of C–Cl makes it far harder to break.
Key points:
Lower bond enthalpy → faster hydrolysis rate
C–I is weakest → hydrolyses fastest
C–F is strongest → essentially no hydrolysis
Experimental results using AgNO₃ confirm this ordering conclusively.
During hydrolysis, a nucleophile such as water or hydroxide ion attacks the δ+ carbon and replaces the halogen to form an alcohol and a halide ion.
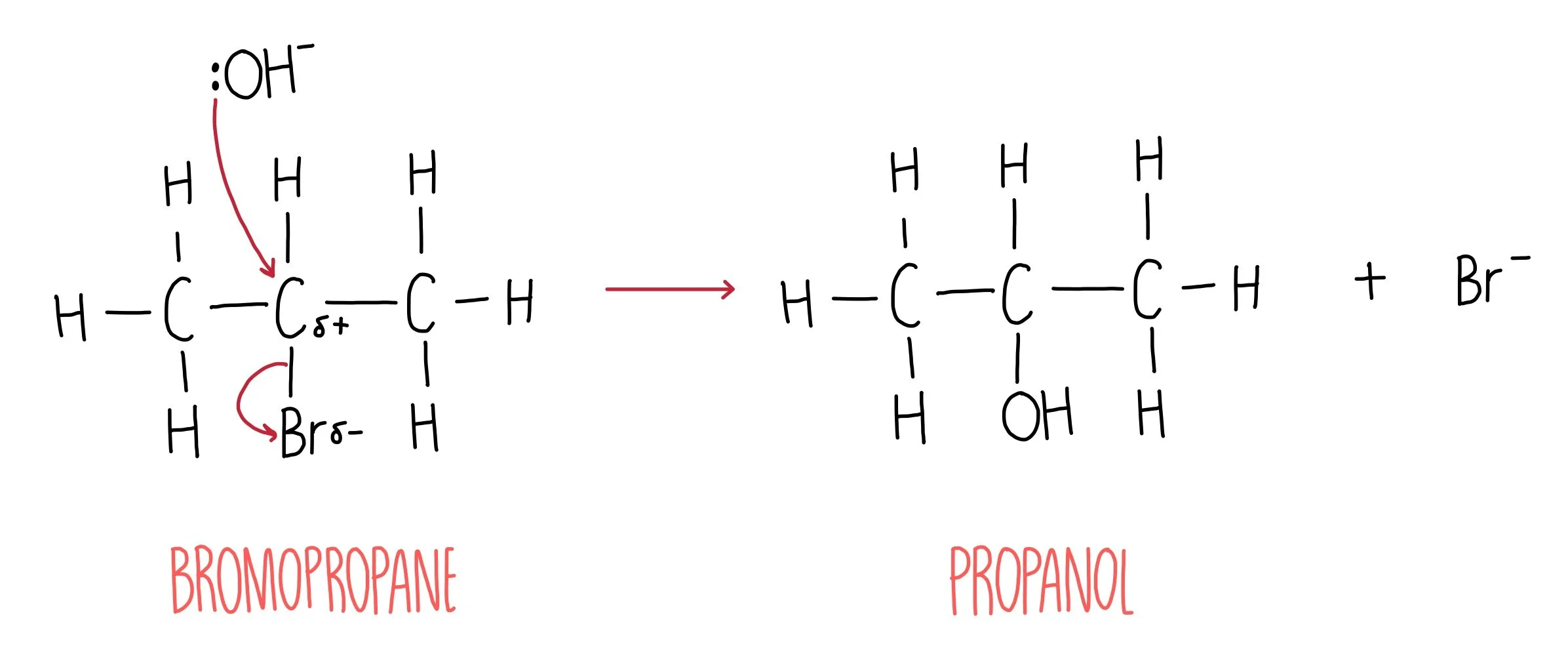
This diagram illustrates nucleophilic substitution where the nucleophile attacks the δ⁺ carbon and the halide leaves, forming an alcohol. It visually reinforces that the rate of this process depends on the strength of the C–X bond, though the curved-arrow electron flow shown is additional mechanism detail not required for this specific subsubtopic. Source
Summary of the Trend
The ordered relationship between bond enthalpy and hydrolysis rate is:
1-iodoalkane: fastest hydrolysis (weakest C–I bond)
1-bromoalkane: moderate hydrolysis
1-chloroalkane: slow hydrolysis
1-fluoroalkane: negligible hydrolysis (strongest C–F bond)
This relationship fulfils the specification requirement to explain hydrolysis rate trends in primary haloalkanes through C–X bond enthalpy differences and to relate them directly to experimental observations.
FAQ
Polarity affects how strongly the carbon atom attracts nucleophiles, but its influence is minor compared with bond enthalpy.
Although C–Cl is more polar than C–I, the C–Cl bond is significantly stronger, so hydrolysis remains slower despite greater polarity.
In primary haloalkanes, bond enthalpy is the dominant factor governing the rate.
Larger halide ions such as iodide can better disperse negative charge, making them more stable once they have left the haloalkane.
As a result, they act as more effective leaving groups.
Smaller halide ions (like chloride) hold charge more tightly, making departure less favourable and slowing hydrolysis.
Ethanol acts as a co-solvent, allowing both the haloalkane (organic) and aqueous silver nitrate (aqueous) to mix effectively.
It prevents phase separation and ensures consistent contact between reactants, improving the reliability of hydrolysis rate comparisons.
Several variables can affect timing measurements:
Inconsistent temperatures slow or accelerate all hydrolysis reactions.
Variation in haloalkane volume or concentration alters the rate.
Impurities or ageing of silver nitrate may affect precipitate formation.
Careful control of these conditions is essential for valid comparisons.
The C–F bond is not only strong but also short, giving exceptionally effective orbital overlap and high bond enthalpy.
Fluoride ions also make extremely poor leaving groups because they retain negative charge tightly.
Together, these factors make breaking the C–F bond thermodynamically and kinetically unfavourable, even with concentrated reagents or high temperatures.
Practice Questions
Explain why 1-iodobutane hydrolyses faster than 1-chlorobutane when heated with aqueous silver nitrate.
(2 marks)
Mark points:
1 mark: C–I bond has a lower bond enthalpy than the C–Cl bond.
1 mark: Weaker C–I bond breaks more easily, so hydrolysis occurs faster.
A student investigates the hydrolysis of three primary haloalkanes: 1-chlorobutane, 1-bromobutane and 1-iodobutane. The student mixes each haloalkane with ethanol and aqueous silver nitrate, then records the time taken for a precipitate to appear.
(a) Explain why a precipitate forms during this reaction.
(b) Using ideas about bond enthalpies, explain the trend in the times recorded for the three haloalkanes.
(c) Identify which silver halide forms last and explain why.
(5 marks)
(a) (2 marks)
1 mark: Hydrolysis releases halide ions.
1 mark: Halide ions react with Ag⁺ ions to form an insoluble silver halide precipitate.
(b) (2 marks)
1 mark: Down the group, the carbon–halogen bond enthalpy decreases (C–Cl strongest; C–I weakest).
1 mark: Weaker bonds break more easily, so the hydrolysis rate increases from chloro- to iodo- compounds.
(c) (1 mark)
1 mark: Silver chloride forms last because the C–Cl bond is strongest and hardest to break, giving the slowest hydrolysis rate.

