OCR Specification focus:
‘Carry out separating funnel extraction to remove layers, dry organic liquids with anhydrous salts such as MgSO4 or CaCl2, and purify by redistillation.’
This section explores essential laboratory techniques for preparing and purifying organic liquids, focusing on extraction, drying, and redistillation to isolate products efficiently and improve sample purity in synthetic chemistry.
Preparation and Purification of Liquids
The preparation and purification of organic liquids forms a core practical skill in A-Level Chemistry. Mastery of these methods allows chemists to isolate products from reaction mixtures, remove impurities and obtain materials suitable for analysis. The OCR specification emphasises three main processes: separating funnel extraction, drying with anhydrous salts, and purification by redistillation. Together, these techniques support accurate characterisation and improve the reliability of synthetic work.
Separation Using a Separating Funnel
A separating funnel is used when two immiscible liquids must be separated following a reaction. This typically involves an organic layer and an aqueous layer, whose differing densities allow physical separation.
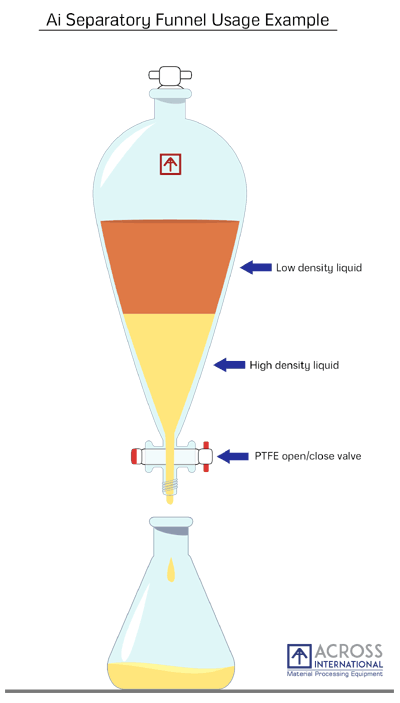
Diagram of a separatory funnel containing two immiscible liquids, illustrating how density determines which layer is drained during liquid–liquid extraction. Source
Place the reaction mixture into the separating funnel and insert the stopper securely.
Invert and shake gently, releasing pressure intermittently by opening the tap.
Allow the mixture to settle so that the layers reform clearly.
Identify the upper and lower layers based on density (organic layers are often, but not always, the upper layer).
Drain the lower layer first, collecting each layer separately in labelled containers.
If required, wash the organic layer with water, dilute acids, or alkalis to remove residual impurities before separation is repeated.
Emulsion formation may occur when vigorous shaking disperses droplets between layers. Gentle mixing and the addition of saturated sodium chloride solution (“salting out”) can help the layers separate more cleanly.
Immiscible liquids: Liquids that do not mix to form a homogeneous solution because intermolecular forces prevent mutual solubility.
Careful identification of the layers is essential, as incorrect separation leads to product loss. Students should note that halogenated organic solvents, such as dichloromethane, are denser than water and therefore form the lower layer.
Drying Organic Liquids
Once separated, the organic layer often contains dissolved water, which must be removed before purification or analysis. Solid anhydrous drying agents remove water by forming solid hydrates.

Photographs demonstrating the use of anhydrous magnesium sulfate to dry an organic liquid and the subsequent gravity filtration used to remove the drying agent. Source
Common drying agents include:
Anhydrous magnesium sulfate (MgSO₄) – fast-acting, suitable for most organic solvents.
Anhydrous calcium chloride (CaCl₂) – effective for hydrocarbons and some chlorinated solvents.
Procedure for drying:
Add a small quantity of drying agent to the organic liquid.
Swirl gently and observe whether the agent clumps (indicating remaining water).
Continue adding small amounts until the solid remains loose and free-flowing.
Allow the mixture to stand to ensure complete removal of moisture.
Filter or decant to remove the drying agent before further purification.
Drying agent: A hygroscopic solid that binds water molecules from an organic liquid to produce a dry solution suitable for purification.
Once dried, the liquid is ready for redistillation to improve purity.
Purification by Redistillation
Redistillation purifies an organic liquid by exploiting differences in boiling points between the desired product and any remaining impurities. It is particularly important when the initial distillation gives a crude product or when high purity is required for analytical techniques such as IR or mass spectrometry.
Key steps in redistillation:
Assemble Quickfit apparatus, including a round-bottom flask, still head, condenser, thermometer, and receiver.
Heat the crude liquid gently so that vapour rises through the still head.
Collect only the fraction that distils at the known boiling point of the desired product.
Discard fractions that distil significantly below or above the target temperature, as these contain impurities or alternative products.
Correct thermometer placement is essential: the bulb should sit just below the side arm of the still head to measure vapour temperature accurately.
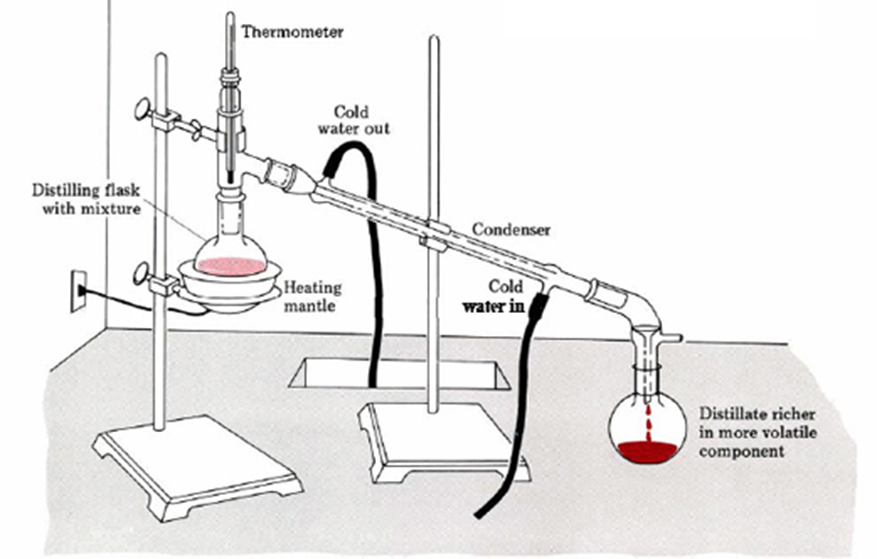
Diagram of a simple distillation setup illustrating the thermometer position, condenser arrangement, and collection of purified distillate during redistillation. Source
Considerations for Effective Purification
Mastery of liquid purification requires understanding of solvent properties, density differences, and boiling point behaviour. Several practical considerations improve outcomes:
Use minimal solvent during extraction to avoid excessive dilution and reduce the time required for drying.
Avoid overheating during distillation, which may lead to bumping or decomposition of heat-sensitive compounds.
Ensure apparatus is airtight to prevent loss of volatile organic vapours.
Choose an appropriate drying agent, as some agents react with alcohols, ketones, or acidic compounds.
Record boiling points carefully, comparing collected fractions with standard literature values.
Integrated Workflow for Purifying Organic Liquids
To bring together the specification-required techniques, a typical workflow for isolating an organic liquid product is:
Transfer reaction mixture to a separating funnel.
Allow layer formation and drain layers sequentially.
Wash organic layer where necessary to remove acidic, basic, or water-soluble impurities.
Add drying agent until the liquid is free of moisture.
Filter off drying agent to obtain a dry crude liquid.
Redistil the liquid to obtain a fraction at the correct boiling point.
Each stage improves the purity and reliability of the final product and is assessed directly through OCR practical tasks.
Importance of Technique Competence in Organic Chemistry
Accurate preparation and purification underpin successful synthetic chemistry. These methods directly support other analytical techniques in Module 4, as pure liquids produce clearer spectra and more reliable data. Understanding the principles behind extraction, drying, and redistillation equips students with essential laboratory competence and aligns closely with the skills expected in the OCR A-Level Chemistry course.
FAQ
The chosen solvent must be immiscible with water and selectively dissolve the organic product while leaving inorganic salts and polar impurities in the aqueous layer.
Other considerations include boiling point (for easy removal later), low toxicity, and chemical inertness to avoid reacting with the product.
Common choices include diethyl ether, cyclohexane, and dichloromethane, depending on density and polarity requirements.
Pressure builds up inside the funnel due to volatile solvents or acid–base reactions producing gases.
If not released, this pressure can force out the stopper or cause solvent spray when the tap is opened.
To avoid this risk:
Invert the funnel carefully
Open the tap away from you to release built-up pressure
Repeat after every shake
Cloudiness usually indicates an emulsion, caused by tiny droplets of one liquid suspended in another.
To resolve this:
Leave the mixture to stand so droplets coalesce
Add saturated sodium chloride solution to “salt out” the phases
Gently swirl rather than shake to avoid re-forming the emulsion
Some drying agents can react with specific functional groups.
For example, calcium chloride may bind alcohols and is unsuitable when drying alcohol-containing products.
Magnesium sulfate is more general-purpose and is preferred for many organic liquids, but it acts quickly and may require multiple additions to reach a free-flowing state.
Purity depends on how narrow the boiling range is and how consistently the thermometer measures vapour temperature.
Key factors include:
Correct thermometer placement at the still head
Slow, controlled heating to prevent co-distillation of impurities
Discarding early and late fractions that fall outside the expected boiling point
Even small errors in heating rate or apparatus setup can broaden the collected fraction and reduce purity.
Practice Questions
A student dries an organic layer using anhydrous magnesium sulfate.
(a) Describe how the student can tell when enough drying agent has been added.
(b) State the final step required after drying the organic layer.
(2 marks)
(a)
Drying agent remains free-flowing / no longer clumps (1)
(b)
Filter or decant the mixture to remove the drying agent (1)
A reaction mixture contains an impure organic liquid product dissolved alongside an aqueous layer.
Describe how the student would separate, dry, and purify the organic liquid using techniques required in the OCR A-Level Chemistry specification.
Your answer should include the principles behind each step and the apparatus involved.
(5 marks)
Award marks for any five of the following points:
Transfer the mixture to a separating funnel and allow the two layers to settle (1)
Identify the organic and aqueous layers based on density and drain off each layer separately (1)
Wash the organic layer if required to remove acidic/basic impurities (1)
Add anhydrous drying agent (e.g. MgSO4 or CaCl2) until it remains free-flowing, indicating water has been removed (1)
Filter or decant to remove the drying agent and obtain a dry crude liquid (1)
Purify the liquid by redistillation, collecting the fraction that distils at the known boiling point of the product (1)
(Max 5 marks)

