OCR Specification focus:
‘Predict likely physical properties and reaction types from the functional groups present, applying knowledge from Module 4 transformations.’
This section introduces how recognising functional groups allows chemists to anticipate key physical properties and probable reaction pathways. Understanding these relationships supports reliable prediction of behaviour in organic molecules.
Predicting Properties from Functional Groups
Functional groups determine how an organic molecule interacts physically and chemically. Their influence on polarity, intermolecular forces, boiling point, volatility, and solubility forms the basis of predicting observable behaviours.
Polarity and Intermolecular Forces
A molecule’s polarity depends on differences in electronegativity and the shape surrounding its functional groups. Polar groups such as –OH, –COOH, and C=O affect how molecules attract one another.
Polarity: Uneven distribution of electron density across a bond or molecule, creating partial positive and negative regions.
Functional groups influence the dominant intermolecular force present:
Hydrogen bonding — occurs in molecules with O–H or N–H groups; leads to higher boiling points and greater solubility in water.
Permanent dipole–dipole interactions — common in carbonyl-containing groups (aldehydes, ketones).
London dispersion forces — found in non-polar species such as alkanes; strength increases with molecular size.
Hydrogen bonding particularly affects alcohols and carboxylic acids, giving them noticeably lower volatility compared with structurally similar hydrocarbons.
Volatility and Boiling Points
Boiling point trends reflect the type and strength of intermolecular forces:
Molecules capable of hydrogen bonding (e.g. alcohols, carboxylic acids) show the highest boiling points among comparable species.
Carbonyl groups produce higher boiling points than alkanes due to dipole–dipole interactions, though lower than those with hydrogen bonding.
Alkanes, being non-polar, exhibit the lowest boiling points and the greatest volatility, with values increasing steadily with chain length.
These trends allow prediction of which liquids evaporate most readily or require greater heating during purification procedures.
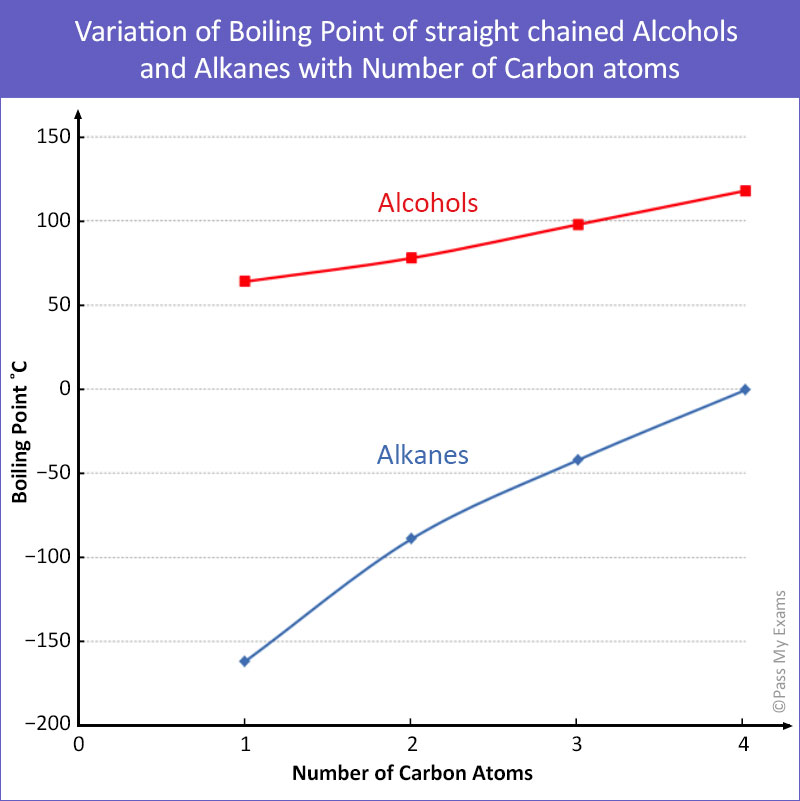
This graph compares the boiling points of straight-chain alcohols and alkanes as carbon number increases. Alcohols have higher boiling points due to hydrogen bonding between –OH groups, while alkanes rely only on London forces. Source
Solubility Behaviour
The presence of polar functional groups influences solubility in water and organic solvents:
Small molecules containing –OH or –COOH groups dissolve well in water due to their ability to form hydrogen bonds with the solvent.
Increasing hydrocarbon chain length reduces solubility because the non-polar region becomes dominant.
Non-polar molecules such as alkenes and alkanes dissolve more readily in non-polar organic solvents.
Short-chain alcohols dissolve readily in water because hydrogen bonds form between the polar –OH group and water molecules.
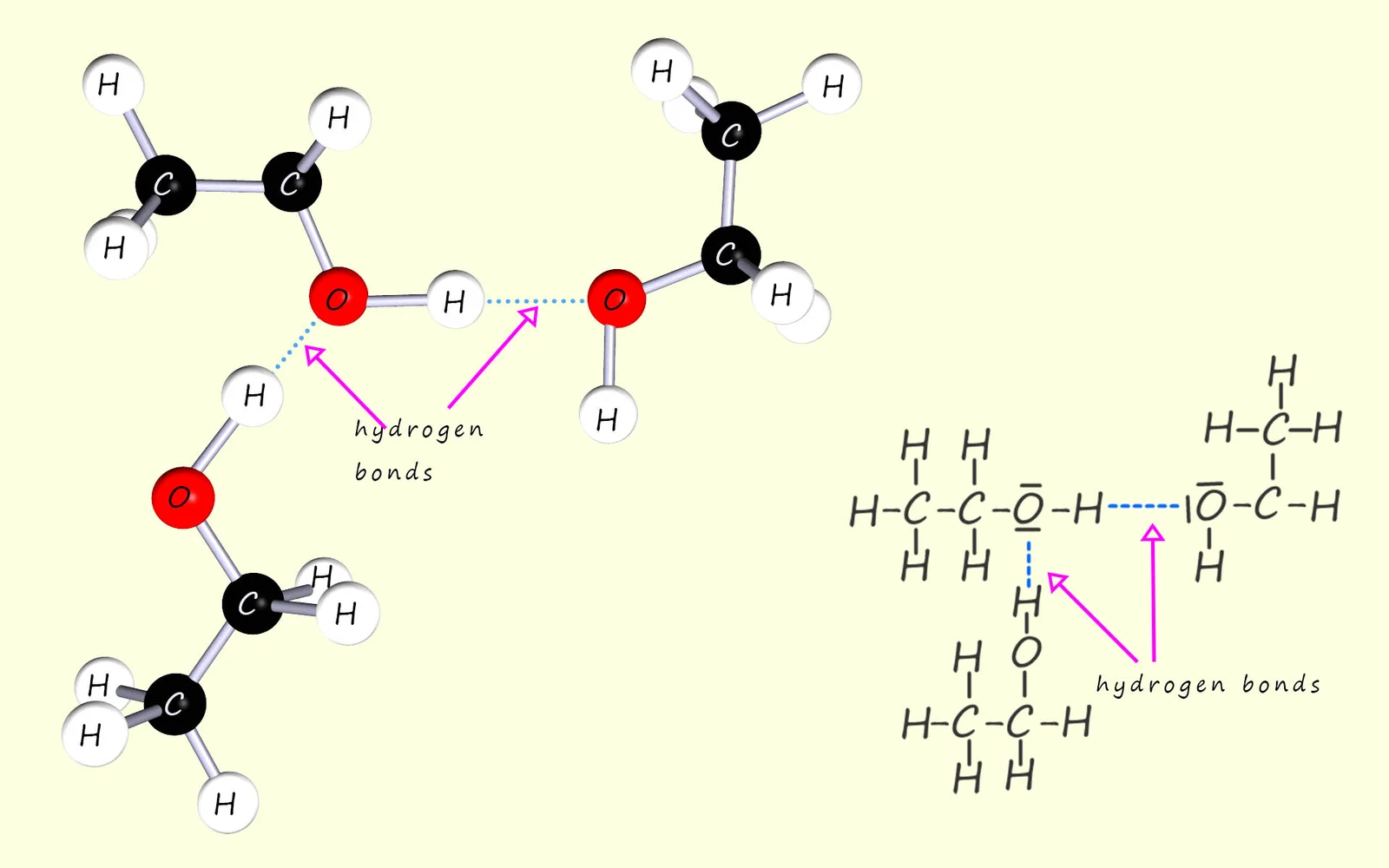
Ethanol molecules form hydrogen bonds with surrounding water molecules via their polar O–H groups. These interactions explain the high solubility of low-molecular-mass alcohols despite their non-polar hydrocarbon regions. Source
Solubility predictions help inform purification approaches such as liquid–liquid extraction.
Predicting Reaction Types from Functional Groups
Each functional group is associated with characteristic reactivity. Predicting reaction pathways is essential in synthetic planning, as highlighted by the OCR specification.
Nucleophilic and Electrophilic Sites
In organic molecules, electron-rich sites act as nucleophiles, whereas electron-poor regions behave as electrophiles.
Nucleophile: An electron-pair donor that attacks electron-deficient centres.
Carbonyl compounds are strongly polar, with the carbon atom carrying a partial positive charge. This makes them reactive toward nucleophiles, producing typical nucleophilic addition reactions.
A normal sentence follows here before the next definition.
Electrophile: A species that accepts an electron pair from a nucleophile during bond formation.
Functional groups involving π-bonds or polar bonds therefore dictate whether a species undergoes addition, substitution, or elimination.
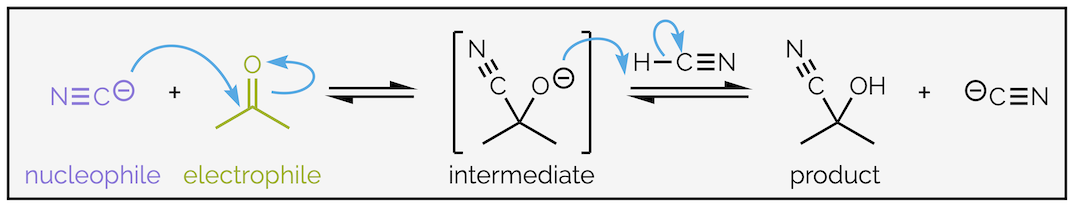
This diagram illustrates electron flow from nucleophiles to electrophilic centres using curved arrows. It highlights how polar bonds and electron density determine reaction types such as nucleophilic addition and substitution. Source
Predicting Reactions by Functional Group
Below are key reaction types associated with common Module 4 functional groups:
Alcohols
Alcohols undergo reactions influenced by the polarity of the O–H bond:
Oxidation (primary → aldehydes → carboxylic acids; secondary → ketones).
Dehydration, forming alkenes in acidic conditions.
Substitution, forming haloalkanes via replacement of the –OH group.
Alkenes
The C=C double bond acts as a region of high electron density, making alkenes susceptible to electrophilic addition. Typical reactions include:
Addition of hydrogen halides.
Hydration to form alcohols.
Halogenation to produce dihaloalkanes.
These additions break the π-bond and form new σ-bonds.
Carbonyl Compounds
Aldehydes and ketones contain a polar C=O group:
Undergo nucleophilic addition (e.g. with H⁻ or CN⁻).
Aldehydes are more reactive due to reduced steric hindrance and less electron donation from surrounding alkyl groups.
Carboxylic Acids and Derivatives
The acidic –COOH group enables:
Acid–base reactions with metals, carbonates, and bases.
Nucleophilic acyl substitution in derivatives such as acyl chlorides and esters.
Their high polarity influences melting and boiling points significantly.
Haloalkanes
Haloalkanes contain a polar C–X bond, making them prone to nucleophilic substitution:
Hydroxide ions form alcohols.
Cyanide ions extend carbon chains.
Ammonia forms amines.
Reaction rate depends on carbon–halogen bond enthalpy, with weaker bonds (e.g. C–I) hydrolysing fastest.
Integrating Functional Groups into Predictive Thinking
By recognising which functional groups are present, students can reliably anticipate:
Whether the molecule is likely to be polar or non-polar.
The predominant intermolecular forces and resulting physical properties.
The most probable reaction mechanisms, such as addition, substitution, oxidation, or elimination.
How structural features influence reactivity patterns.
Bullet points and structured reasoning support clear decision-making in synthetic planning, fully aligning with the OCR requirement to predict physical properties and reaction types from functional groups.
FAQ
Branching reduces the surface area of molecules, which weakens London dispersion forces between them.
As a result, branched molecules usually have lower boiling points than straight-chain isomers, even when the same functional group is present. This effect must be considered alongside functional group polarity when predicting volatility.
Aldehydes have one alkyl group attached to the carbonyl carbon, whereas ketones have two.
This leads to:
Less steric hindrance around the carbonyl carbon
A greater partial positive charge on the carbon atom
Both factors make aldehydes more susceptible to nucleophilic attack than ketones.
Functional groups determine whether a molecule can donate a proton.
For example:
Carboxylic acids contain a –COOH group that can release H⁺ ions
Alcohols are much weaker acids due to less stabilisation of the conjugate base
Alkanes and alkenes are effectively neutral
This allows acidity to be predicted from structure alone.
Haloalkanes contain a polar C–X bond but no multiple bonds.
The carbon atom bonded to the halogen is electron-deficient, making it vulnerable to nucleophilic attack. However, the absence of a π bond means addition reactions are not favoured, unlike in alkenes.
Highly symmetrical molecules tend to pack more efficiently in the solid state.
This can result in:
Higher melting points
Stronger intermolecular attractions in solids
Symmetry does not usually affect reactivity directly, but it is useful when predicting physical behaviour from molecular structure.
Practice Questions
Ethanol and ethane have similar molecular sizes.
Explain why ethanol has a higher boiling point than ethane.
(2 marks)
Award marks as follows:
1 mark for stating that ethanol can form hydrogen bonds due to the presence of an O–H group.
1 mark for explaining that hydrogen bonding is stronger than the London dispersion forces present in ethane, requiring more energy to separate molecules.
Maximum 2 marks.
An organic compound contains a carbonyl (C=O) functional group and has the molecular formula C3H6O.
a) Predict one physical property of this compound and explain your answer. (2 marks)
b) Predict one type of reaction this compound is likely to undergo and explain your reasoning in terms of bonding and electron distribution. (3 marks)
(5 marks)
a) Physical property prediction (2 marks)
1 mark for correctly predicting a relevant physical property, e.g. relatively high boiling point or moderate solubility in water.
1 mark for explaining that the polar C=O bond leads to permanent dipole–dipole interactions between molecules.
b) Reaction type prediction (3 marks)
1 mark for identifying a suitable reaction type, e.g. nucleophilic addition.
1 mark for stating that the C=O bond is polar, with a partially positive carbon atom.
1 mark for explaining that this electron-deficient carbon can be attacked by a nucleophile due to electron pair donation.
Maximum 5 marks.

