OCR Specification focus:
‘Identify functional groups present in an organic molecule drawn from those encountered in Module 4 to predict reactions and properties.’
Organic molecules exhibit characteristic atoms or groups of atoms that determine their behaviour. Recognising functional groups is essential for predicting physical properties and the types of reactions an organic compound will undergo.
Identifying Functional Groups in Organic Molecules
Functional groups are recurring structural features in organic compounds. They provide the basis for classification and determine much of a molecule’s chemistry. OCR expects students to identify these groups in unfamiliar structures drawn from Module 4 content.
Before exploring common groups, it is important to clarify the term functional group.
Functional group: A specific atom or group of atoms in an organic molecule responsible for its characteristic reactions.
Functional groups influence intermolecular forces, boiling points, solubility, and typical reaction mechanisms. When given a displayed, skeletal or structural formula, identifying these features allows accurate predictions of reactivity patterns.
A wide range of functional groups has been introduced in Module 4. Students should be able to recognise each group quickly and associate it with its characteristic transformations.
Recognising Hydrocarbon-Based Groups
Hydrocarbon functional groups form the foundation of many molecules and influence reactivity through bond type and saturation.
Alkanes
Alkanes contain only C–C and C–H single bonds. Their lack of polar bonds means they undergo limited reactions, typically radical substitution in the presence of UV light.
Alkenes
Alkenes include at least one C=C double bond, introducing a site of high electron density. This bond undergoes electrophilic addition, where the π-bond is broken and new atoms are added.
Aromatic Rings
A benzene ring is represented as a six-membered ring with a circle or alternating double bonds. Aromatic rings show characteristic electrophilic substitution reactions due to their stable delocalised π-system.
Recognising Oxygen-Containing Groups
Alcohols
Alcohols contain a hydroxyl group (–OH) attached to a saturated carbon.
Their presence typically increases water solubility and polarity. Alcohols undergo oxidation, substitution, and dehydration reactions depending on structure.
Carbonyl Compounds
Carbonyl groups contain a C=O bond, giving rise to two major families:
Aldehydes: Carbonyl group at the end of a chain (–CHO).
Ketones: Carbonyl group within a chain (–CO–).
The C=O bond is polar, generating susceptibility to nucleophilic addition.
Carboxylic Acids
Carboxylic acids feature a carboxyl group (–COOH). Their highly polar structure allows hydrogen bonding and typical acid–base behaviour.
Carboxyl group: A functional group containing a carbonyl and hydroxyl bonded to the same carbon, written as –COOH.
Carboxylic acids also form esters and acyl derivatives, linking structure to reactivity.
Esters
Esters contain the functional group –COO–, produced from reactions between carboxylic acids and alcohols. They are common in synthesis pathways and are identifiable by the C–O–C linkage adjacent to a carbonyl.
Recognising Halogen-Containing Groups
Haloalkanes
Haloalkanes possess at least one carbon–halogen bond (C–X), where X = F, Cl, Br or I. These bonds vary in polarity and strength, influencing their reactivity.
Haloalkanes typically undergo nucleophilic substitution reactions, allowing conversion into a range of other functional groups such as alcohols and amines.
Applying Functional Group Identification to Predict Chemistry
Recognising functional groups is not simply an exercise in pattern-spotting. OCR emphasises their use in predicting reactions and physical properties. When analysing an organic structure, students should look for:
1. Key structural features
Presence of multiple bonds (C=C, C≡C, C=O)
Presence of heteroatoms (O, N, halogens)
Arrangement of atoms (end-chain or within chain)
Overall shape (cyclic, aromatic, branched)
2. Associated reactivity patterns
Each functional group is linked to certain reaction types:
Nucleophilic substitution → haloalkanes
Electrophilic addition → alkenes
Oxidation → primary and secondary alcohols
Nucleophilic addition → aldehydes and ketones
Acid–base reactions → carboxylic acids
Esterification → carboxylic acids and alcohols
A normal sentence is written here to ensure appropriate spacing before the next section.
3. Physical property predictions
Functional groups dictate intermolecular forces such as hydrogen bonding, permanent dipole–dipole interactions, and van der Waals forces. These influence:
Volatility
Melting and boiling points
Solubility in water or organic solvents
Functional Group Identification Strategy
Students should develop a systematic approach when examining unfamiliar molecules:
Step-by-step method
Scan the structure for double or triple bonds.
Identify any heteroatoms and their bonding patterns.
Match patterns to known Module 4 functional groups.
Consider whether multiple functional groups are present, as these affect reaction pathways.
Link each identified group to a familiar set of reactions.
Structural formula interpretations
Displayed, structural and skeletal formulae all show functional groups in different ways. Skeletal formulae often require particular care, as heteroatoms and functional groups must be recognised without explicit carbon or hydrogen labelling.
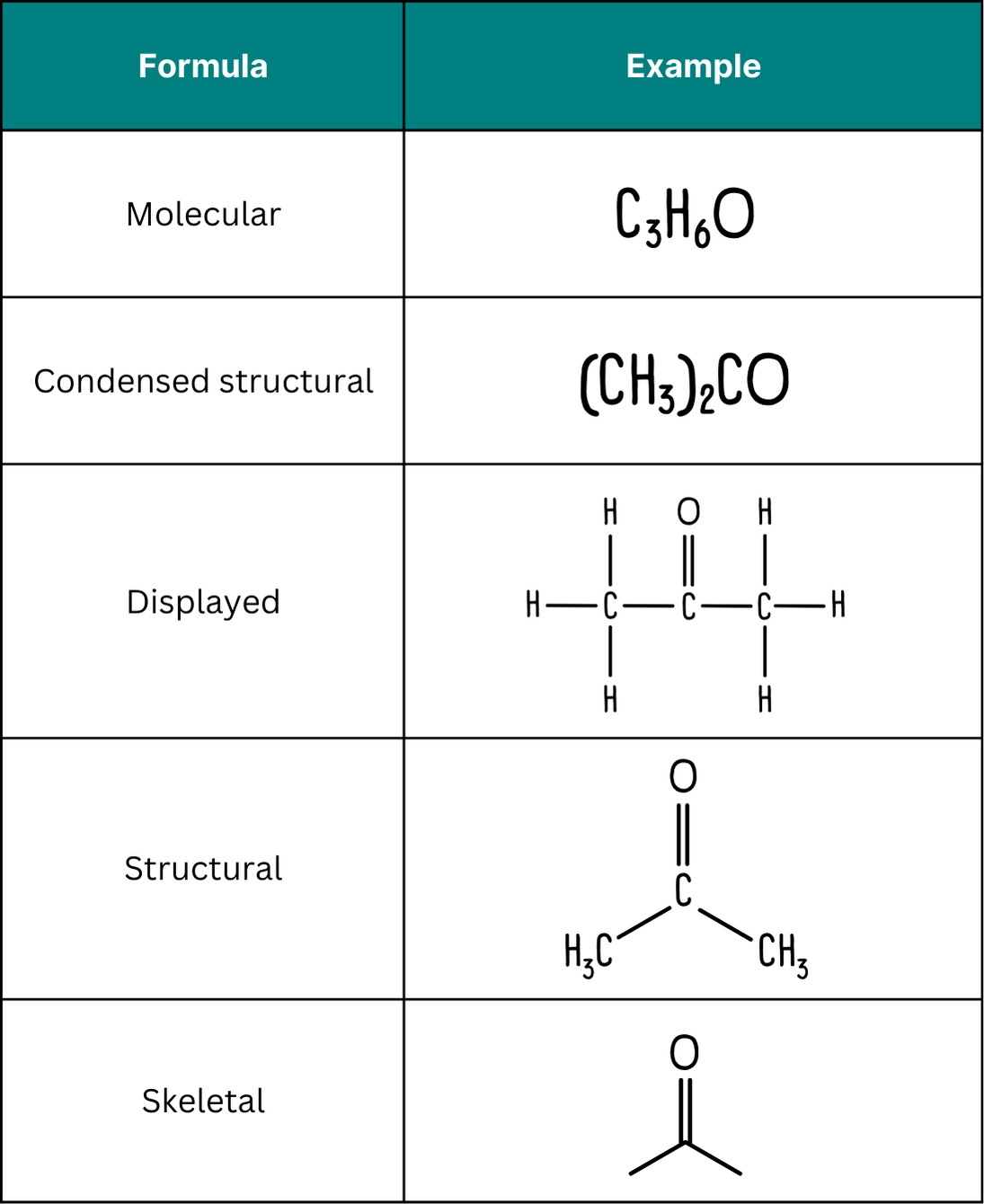
This image compares different representations of the same molecule, highlighting how the carbonyl functional group appears consistently across formula types, supporting accurate identification in varied formats. Source
Importance in Organic Synthesis
Identifying functional groups allows students to map out transformations between molecules. Many Module 4 reactions are specific to certain groups, making accurate recognition essential for designing synthetic routes.
Correct identification enables prediction of feasible reaction sequences, selection of appropriate reagents, and understanding of likely reaction conditions.
In summary, functional group recognition underpins organic chemistry, guiding understanding of reactivity, mechanisms and physical behaviour—skills central to mastering Module 4 and the wider OCR A-Level Chemistry course.
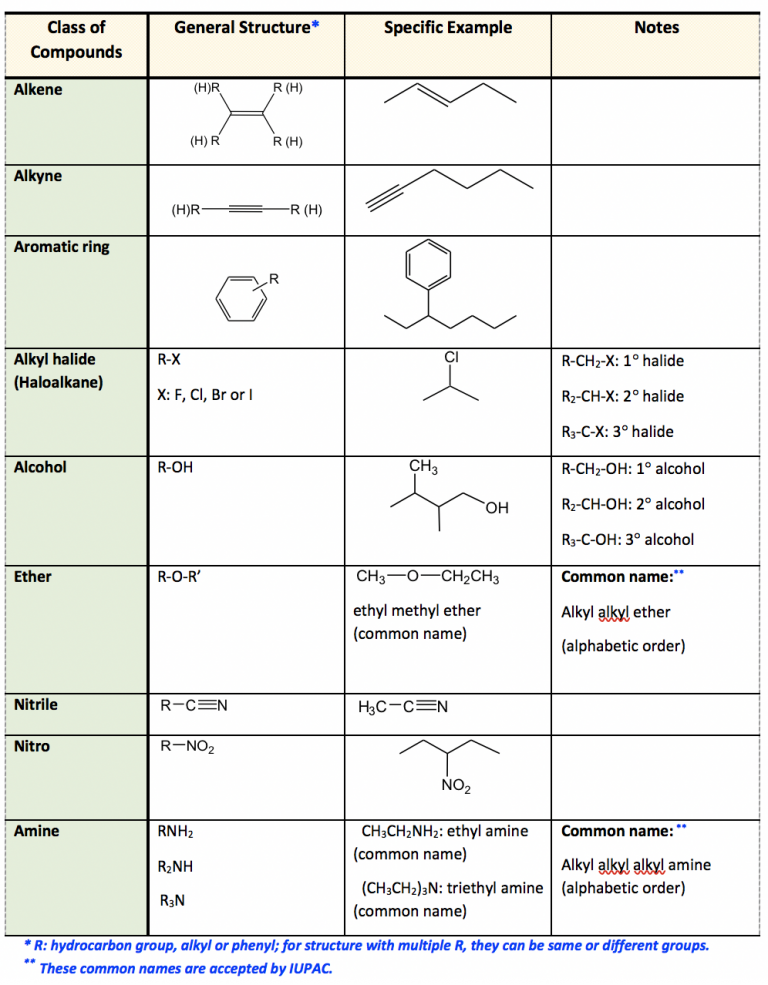
This table summarises key functional groups with representative structures, reinforcing recognition of common patterns such as R–OH and R–COOH, though it includes some groups beyond the OCR specification. Source
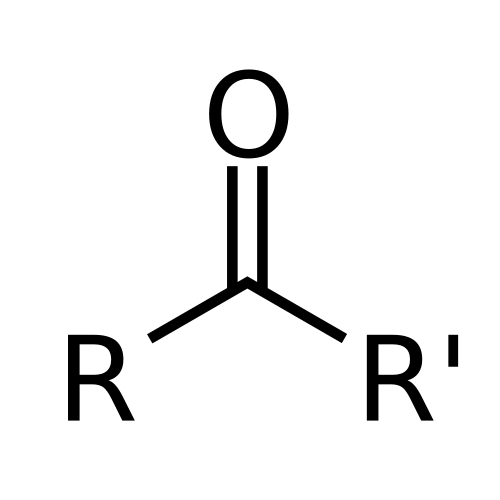
This diagram illustrates the ketone functional group, emphasising the central C=O bond and the variable R groups, supporting recognition of carbonyl-containing functional groups. Source
FAQ
Functional groups affect electron distribution within a molecule, altering how readily it donates or accepts protons.
Acidic behaviour increases when a functional group stabilises the negative charge formed after proton loss, as seen in carboxylic acids.
Basicity is linked to the presence of lone pairs, such as those on oxygen in alcohols, which can act as weak bases but are far less basic than amines.
Each bond type vibrates at a characteristic frequency determined by mass and bond strength.
For example:
C=O bonds absorb strongly near 1700 cm–1 due to high bond polarity.
O–H bonds show broad absorptions from hydrogen bonding.
These patterns allow quick identification of functional groups beyond simple structural inspection.
When a molecule contains more than one functional group, each may undergo different reactions under the same conditions.
Reactivity is often prioritised by:
Functional group susceptibility (e.g., carbonyl vs alkene).
Electrophilicity or nucleophilicity of specific sites.
Steric influences around reactive centres.
Recognising all groups allows more accurate mapping of feasible transformations.
Resonance delocalises electron density across multiple atoms, stabilising functional groups and altering their typical reactivity.
Carboxylic acids and aromatic rings both exhibit resonance, which:
Enhances acidity (carboxylate ion stabilisation).
Directs electrophilic substitution in aromatic systems.
Resonance effects must be considered when predicting how a functional group behaves in reactions.
Skeletal formula simplifies structures by removing carbon and hydrogen labels, making functional groups stand out more clearly.
This format highlights:
Position of heteroatoms
Double and triple bonds
Branching and ring systems
Skeletal representation is particularly useful for analysing large molecules or those containing repeated structural motifs.
Practice Questions
The structure of an organic molecule is shown below:
CH3–CH2–CH2–OH
a) Identify the functional group present.
b) State one typical reaction this functional group can undergo.
(2 marks)
a) Identification of the functional group:
Correctly states alcohol / hydroxyl group (–OH). (1 mark)
b) Typical reaction:
States oxidation, substitution, or dehydration to form an alkene. (1 mark)
A chemist analyses an unknown organic compound with the structural formula:
CH3–CH2–CO–CH3
a) Identify the functional group present in this molecule.
b) Explain how you can distinguish this functional group from an aldehyde using structural features alone.
c) The chemist predicts that this compound cannot be oxidised under standard laboratory conditions. Explain why, using your understanding of functional group behaviour.
(5 marks)
a) Identification of functional group:
Correctly states ketone / carbonyl group. (1 mark)
b) Distinguishing from an aldehyde:
Any one of the following for each marking point:
States that the carbonyl group is within the carbon chain. (1 mark)
States that aldehydes have a carbonyl at the end of the chain (–CHO), whereas ketones have R–CO–R. (1 mark)
c) Explanation of lack of oxidation:
States ketones cannot be oxidised under normal laboratory conditions. (1 mark)
Explains this is because oxidation would require breaking a C–C bond, which is not feasible with common oxidising agents. (1 mark)
Total: 5 marks

