OCR Specification focus:
‘Apply transformations between Module 4 functional groups; integrate additional reactions supplied in assessments to extend synthetic planning skills.’
This topic develops the ability to apply known organic reactions systematically, selecting and combining transformations to plan efficient synthetic routes between functional groups.
Purpose of a Reaction Toolkit
In organic synthesis, students are expected to apply a reaction toolkit rather than memorise isolated reactions. This means recognising which familiar transformations can be used to convert one functional group into another and combining them logically. The emphasis is on application, not recall, and on using reactions already encountered in Module 4.
A reaction toolkit consists of:
Known functional group interconversions
Typical reagents and conditions
Awareness of reaction limitations and compatibility
Logical sequencing of reactions to reach a target molecule
Functional Groups within the Toolkit
Only functional groups encountered in Module 4 are relevant. These include:
Alkanes
Alkenes
Haloalkanes
Alcohols
Aldehydes
Ketones
Carboxylic acids
Students must recognise how these groups relate to one another through common reactions such as oxidation, reduction, substitution, elimination, and hydrolysis.
Core Transformations to Apply
When applying the toolkit, transformations should be selected from familiar chemistry rather than invented.
Oxidation Reactions
Oxidation reactions are commonly used to increase the oxidation state of carbon.
Primary alcohols can be oxidised to aldehydes or further to carboxylic acids
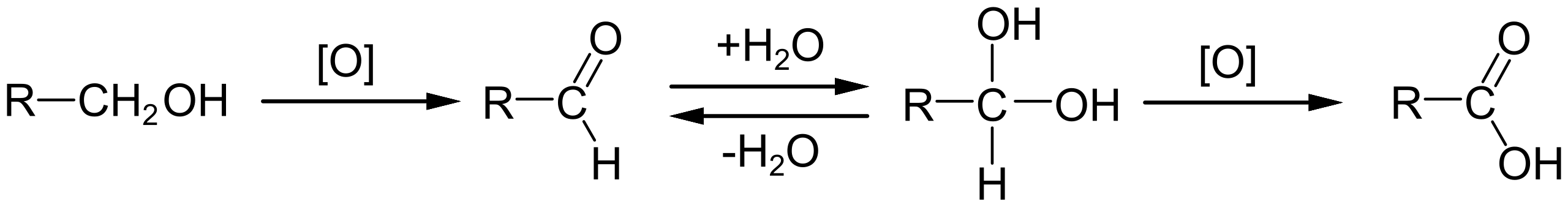
This scheme summarises the oxidation sequence for a primary alcohol, showing stepwise conversion to an aldehyde and then to a carboxylic acid. It illustrates controlled oxidation as a deliberate functional group interconversion in synthesis. Source
Secondary alcohols oxidise to ketones
Tertiary alcohols are resistant to oxidation
Control of conditions is essential, as over-oxidation can occur if unsuitable reagents or heating are used.
Reduction Reactions
Reduction allows conversion to more reduced functional groups.
Alkenes can be reduced to alkanes
Aldehydes and ketones can be reduced to alcohols
Reductions are often used to reverse oxidation steps or to finalise a synthetic route.
Substitution Reactions
Substitution reactions enable functional group exchange.
Haloalkanes can be converted into alcohols via nucleophilic substitution
Alcohols can be converted back into haloalkanes using halide ions and acid
These reactions are useful for introducing or removing halogen atoms during synthesis.
Elimination Reactions
Elimination reactions remove small molecules to form multiple bonds.
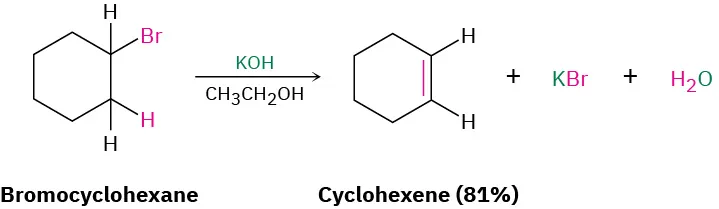
This diagram shows an elimination reaction where a haloalkane forms an alkene under basic ethanolic conditions. The percentage yield shown is extra detail beyond OCR requirements, but the functional group change is directly relevant. Source
Alcohols can be dehydrated to form alkenes
Haloalkanes can undergo elimination to produce alkenes under suitable conditions
Elimination is often used to create unsaturation that can be exploited in later steps.
Planning Multi-Step Routes
Applying the toolkit requires planning multi-stage routes rather than single reactions. Students should:
Identify the starting functional group
Identify the target functional group
Work stepwise, selecting known reactions to bridge the gap
Each step must be chemically realistic and based on reactions already learned.
Reaction pathway: A logical sequence of chemical reactions used to convert a starting material into a desired product.
There should always be at least one clear functional group change at each stage.
Sequencing and Compatibility
Correct sequencing matters in synthetic planning.
Some functional groups may not survive harsh conditions
Oxidation should not be attempted after reduction if it reverses progress
Certain reagents may interfere with existing functional groups
Students should avoid unnecessary steps and choose the most direct route possible using known chemistry.
Using Supplied Reactions in Assessments
Examination questions may include additional reactions not previously encountered. These will always be provided with sufficient information.
Students must integrate the new reaction into their existing toolkit
The unfamiliar reaction should be treated as another valid transformation
No memorisation of unfamiliar mechanisms is required
This tests adaptability rather than recall.
Applying Chemical Logic
When applying the reaction toolkit, answers should show clear chemical reasoning:
Each step should be justified by a known transformation
Reagents and conditions should be appropriate for the functional group
Routes should be efficient and chemically sensible
Oxidation–Reduction Relationship = Change in functional group oxidation state
Oxidation = Increase in carbon–oxygen bonding
Reduction = Decrease in carbon–oxygen bonding
This principle helps students track changes across multi-stage routes.
Common Pitfalls to Avoid
Introducing reactions outside Module 4
Skipping steps without chemical justification
Using conditions incompatible with the functional group present
Reversing progress by undoing previous transformations
Applying the reaction toolkit successfully requires structured thinking, familiarity with transformations, and disciplined use of the chemistry already learned.
FAQ
Unnecessary steps increase the risk of side reactions, reduce overall yield, and complicate purification.
In exam questions, efficient routes demonstrate strong chemical understanding. Shorter pathways using familiar transformations are usually preferred, provided all steps are chemically valid.
A realistic pathway only uses reactions encountered in Module 4 and applies them under suitable conditions.
Each step should produce a functional group that is stable under the conditions used in the following step, without undoing earlier transformations.
Unfamiliar reactions test your ability to integrate new information logically rather than recall.
You are expected to treat the supplied reaction as an additional toolkit option and combine it sensibly with reactions you already know.
Some functional groups may react unintentionally under certain conditions.
For example:
Strong oxidising conditions may damage sensitive groups
Acidic conditions may interfere with substitution or elimination steps
Considering compatibility helps prevent chemically impossible routes.
You should clearly show:
The sequence of functional group changes
Appropriate reagents or conditions
Detailed mechanisms are not required unless specifically asked, but vague statements without chemical justification will not gain full credit.
Practice Questions
A student wants to convert a secondary alcohol into a ketone as part of a synthetic route.
a) State the type of reaction used.
b) Name a suitable reagent or reagent system.
(2 marks)
Oxidation stated (1 mark)
Suitable oxidising agent named, e.g. acidified potassium dichromate(VI) or K2Cr2O7/H2SO4 (1 mark)
A student is given an alcohol as a starting material and asked to synthesise a carboxylic acid using reactions from the Module 4 reaction toolkit.
Describe a suitable multi-step reaction pathway. Your answer should include the key functional group changes and appropriate reagents or conditions for each step.
(5 marks)
Recognition that the alcohol must be a primary alcohol to form a carboxylic acid (1 mark)
Identification of oxidation as the required transformation (1 mark)
Correct intermediate identified as an aldehyde or clear indication of controlled then further oxidation (1 mark)
Suitable oxidising agent named, e.g. acidified potassium dichromate(VI) (1 mark)
Appropriate conditions stated or implied, e.g. reflux for full oxidation to carboxylic acid (1 mark)
Maximum 5 marks

