OCR Specification focus:
‘Devise two-stage synthetic routes between functional groups encountered so far, applying given or familiar transformations to reach a target molecule.’
Organic synthesis involves planning logical reaction sequences to convert one functional group into another using known chemical transformations efficiently and selectively under appropriate conditions.
Understanding Two-Stage Synthetic Routes
Two-stage synthetic routes involve converting a starting functional group into a target functional group through two consecutive reactions, each using reagents and conditions specified within the OCR A-Level Chemistry syllabus. Students are expected to recognise suitable intermediates and select reactions already encountered in Module 4.
A successful route requires:
Identification of the starting functional group
Identification of the target functional group
Selection of a sensible intermediate that can be formed and then transformed
Use of appropriate reagents and conditions for each stage
What Is a Synthetic Route?
Synthetic route: A planned sequence of chemical reactions used to convert a starting compound into a desired product via one or more intermediates.
Between each stage, the functional group must change in a chemically realistic way. Reactions must be familiar and syllabus-approved, such as oxidation, reduction, substitution, elimination, or hydrolysis.
Identifying Starting Materials and Targets
The first step in devising a route is analysing the functional groups present in both the starting compound and the target molecule. This determines what transformations are needed.
Key considerations include:
Whether the carbon skeleton stays the same
Whether oxidation level increases or decreases
Whether a functional group needs to be replaced or rearranged
For example:
Alcohol → carboxylic acid involves oxidation
Haloalkane → alcohol involves hydrolysis
Alcohol → alkene involves elimination
Recognising these links allows the selection of an appropriate intermediate that bridges the two stages.
Choosing a Suitable Intermediate
The intermediate must be:
A compound that can realistically be formed from the starting material
Capable of being converted into the final product using known reactions
Common intermediates include:
Alcohols (central to many routes)
Haloalkanes
Alkenes
Aldehydes or ketones
Alcohols are especially important because they can undergo oxidation, substitution, elimination, and combustion, making them versatile intermediates.
Before choosing reagents, plan the route by working backwards from the target functional group to a plausible intermediate, then convert that intermediate to the required starting material.
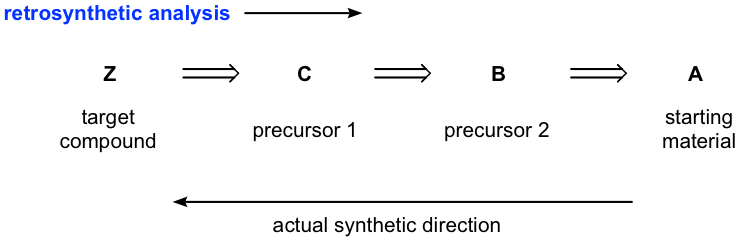
This diagram illustrates retrosynthetic analysis, where the target molecule is simplified stepwise to plausible precursors. The forward synthesis is then written in the opposite direction using appropriate reagents and conditions. Source
Applying Familiar Transformations
Only transformations already encountered in the specification should be used. These include:
Oxidation Reactions
Primary alcohols oxidised to aldehydes or carboxylic acids
Secondary alcohols oxidised to ketones
Tertiary alcohols resist oxidation
Oxidation reactions often use acidified potassium dichromate(VI), with conditions determining the final product.
Substitution Reactions
Alcohols converted to haloalkanes using halide ions and acid
Haloalkanes hydrolysed to alcohols using aqueous alkali
These reactions allow movement between alcohols and haloalkanes depending on the desired outcome.
Elimination Reactions
Alcohols dehydrated to form alkenes
Requires heat and an acid catalyst such as phosphoric acid
Elimination is useful when introducing unsaturation before further reactions.
One common two-stage pattern is alcohol → alkene → product, using dehydration to create an alkene intermediate that can be transformed in the next step.
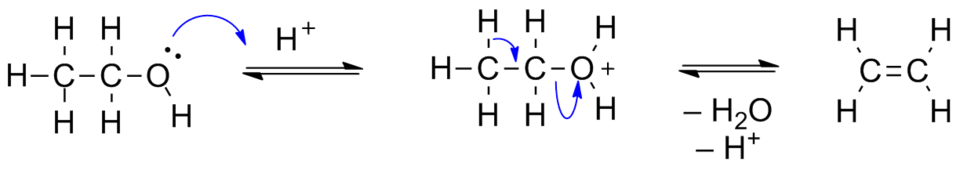
This scheme shows dehydration of an alcohol to form an alkene under acidic conditions. Curly arrows indicating electron movement are included as extra detail beyond OCR requirements, but the functional group change is the key focus. Source
Writing Two-Stage Routes Clearly
Routes should be written as two separate steps, each clearly labelled with reagents and conditions. The intermediate must be shown or named between the steps.
For clarity:
Each stage should show one reaction arrow
Reagents should be written above the arrow
Conditions such as heat, reflux, or catalyst should be included where required
Students are not required to include mechanisms unless explicitly asked elsewhere.
Avoiding Common Errors
When devising routes, several common mistakes should be avoided:
Using reactions not in the specification
Attempting to change more than one functional group in a single step
Choosing an intermediate that cannot undergo the second reaction
Forgetting essential conditions such as aqueous or alcoholic solvents
Routes must be chemically sensible and based on realistic laboratory chemistry.
Linking Routes to Functional Group Properties
Understanding functional group behaviour helps justify route choices. For example:
Alcohols are reactive due to the polar O–H bond
Haloalkanes undergo nucleophilic substitution because of the polar C–X bond
Alkenes undergo addition reactions due to the C=C bond
These properties explain why certain transformations are feasible within two stages.
In two-stage planning, oxidation can be used to move between primary alcohols, aldehydes, and carboxylic acids, with conditions chosen to control how far oxidation proceeds.
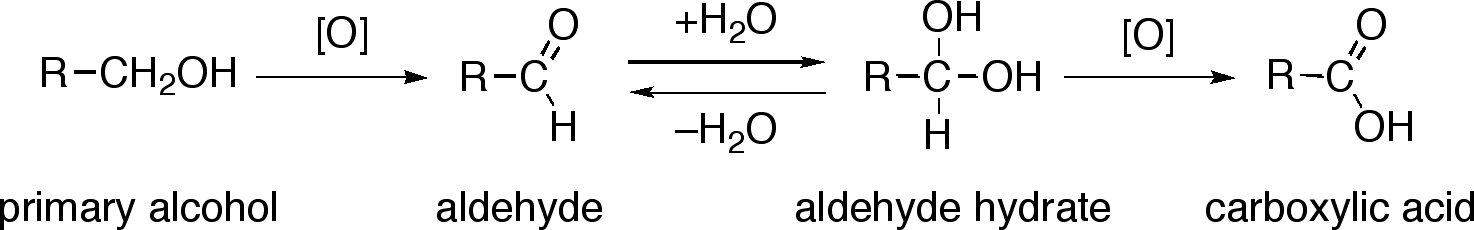
This diagram summarises oxidation of a primary alcohol through an aldehyde to a carboxylic acid. The aldehyde hydrate shown is extra detail beyond OCR requirements but helps explain why aqueous conditions allow further oxidation. Source
Using Given Information in Assessments
In examinations, additional reactions or reagents may be provided. These can be incorporated into routes even if not memorised, provided they are clearly stated in the question.
Students should:
Carefully read all supplied reaction information
Integrate new reactions logically with known transformations
Maintain clear, stepwise routes with justified intermediates
This skill demonstrates an ability to apply chemical knowledge flexibly rather than relying on memorisation alone.
Scope of Two-Stage Routes in OCR Chemistry
Two-stage routes are limited to functional groups encountered so far in Module 4, including alcohols, haloalkanes, alkenes, aldehydes, ketones, and carboxylic acids. More complex synthesis planning is developed later but builds directly on these foundational skills.
Mastering two-stage routes strengthens understanding of reaction chemistry, reinforces functional group interconversion, and prepares students for integrated organic synthesis questions across the specification.
FAQ
The decision depends on which functional group must be present for the second reaction to occur.
If a later reaction requires an alcohol (e.g. oxidation or dehydration), substitution to form the alcohol must come first.
If oxidation would destroy or prevent a later substitution, oxidation should be delayed until the final stage.
Always choose the order that keeps the intermediate chemically stable and reactive under the required conditions.
Alcohols are highly versatile and can undergo several syllabus-approved reactions.
They can:
Be oxidised to aldehydes, ketones, or carboxylic acids
Be dehydrated to form alkenes
Be substituted to form haloalkanes
This flexibility makes alcohols suitable “junction points” when planning efficient two-stage routes.
Yes, more than one valid route may exist.
For example, a carboxylic acid could be formed via:
Alcohol → aldehyde → carboxylic acid
Haloalkane → alcohol → carboxylic acid
As long as both steps use known reactions and correct conditions, alternative intermediates are acceptable in exam answers.
Reaction conditions determine how far oxidation proceeds.
For primary alcohols:
Gentle heating and distillation favour aldehyde formation
Heating under reflux promotes further oxidation to carboxylic acids
Incorrect conditions may lead to over-oxidation, producing the wrong final functional group.
Reagents should be specific enough to show clear chemical understanding.
For example:
“Aqueous sodium hydroxide” is better than “alkali”
“Acidified potassium dichromate(VI)” is better than “oxidising agent”
Precise naming helps examiners identify the intended reaction and award marks accurately.
Practice Questions
A student wants to convert a primary alcohol into a carboxylic acid using a two-stage synthetic route.
(a) Name a suitable intermediate formed in the first stage.
(b) State the type of reaction used in the second stage to form the carboxylic acid.
(2 marks)
(a) Suitable intermediate named
Aldehyde
Award 1 mark
(b) Correct reaction type stated
Oxidation
Award 1 mark
Devise a two-stage synthetic route to convert a haloalkane into a carboxylic acid.
Your answer should:
Name the intermediate formed
State the reagents and conditions used in each stage
Identify the type of reaction occurring in each stage
No reaction mechanisms are required.
(5 marks)
Stage 1: Conversion of haloalkane to alcohol
Correct intermediate identified as an alcohol
Award 1 markCorrect reagents and conditions stated (e.g. aqueous sodium hydroxide, heat/reflux)
Award 1 markReaction type correctly identified as nucleophilic substitution or hydrolysis
Award 1 mark
Stage 2: Conversion of alcohol to carboxylic acid
Correct reagents and conditions stated (e.g. acidified potassium dichromate(VI), heat under reflux)
Award 1 markReaction type correctly identified as oxidation
Award 1 mark
Maximum 5 marks

