OCR Specification focus:
‘Explain absorption of IR by atmospheric gases with C=O, O–H and C–H bonds and the link to global warming that informs energy-use policies.’
Infrared absorption by atmospheric gases depends on molecular bonding and vibration, playing a central role in the greenhouse effect and influencing scientific understanding of global warming.
Infrared Radiation and Molecular Vibrations
Infrared (IR) radiation is part of the electromagnetic spectrum with wavelengths longer than visible light. In chemistry, IR radiation is significant because it interacts with molecules by increasing the vibration of covalent bonds. Not all molecules absorb IR radiation; absorption depends on changes in bond polarity during vibration.
When IR radiation passes through a gas, specific wavelengths are absorbed if they match the natural vibrational frequencies of bonds within the molecules. These absorbed frequencies correspond to energy transfers that increase vibrational motion, such as stretching or bending of bonds.
For atmospheric gases, this absorption of IR radiation is crucial because Earth emits IR radiation as it loses heat to space. Gases that absorb this outgoing IR radiation contribute directly to warming of the atmosphere.
Bonds Responsible for IR Absorption
Only certain covalent bonds absorb IR radiation effectively. The OCR specification highlights three key bond types that are especially relevant in atmospheric gases: C=O, O–H, and C–H bonds.
Polar Bonds and IR Activity
IR absorption requires a change in dipole moment during vibration. Bonds that are polar, or become temporarily polar when vibrating, are IR-active.
Dipole moment: A measure of charge separation in a bond or molecule due to differences in electronegativity between atoms.
C=O bonds are strongly polar because oxygen is much more electronegative than carbon.
O–H bonds are highly polar and show strong, broad IR absorptions.
C–H bonds are less polar but still absorb IR radiation, particularly at higher frequencies.
These bonds are common in many atmospheric gases, especially greenhouse gases, making them effective at absorbing IR radiation emitted from Earth’s surface.
Atmospheric Gases and Their IR Absorption
Different atmospheric gases absorb IR radiation to varying extents depending on their molecular structure.
Carbon Dioxide (CO₂)
Carbon dioxide contains two C=O bonds. These bonds absorb IR radiation strongly at characteristic wavelengths. Although CO₂ is present in relatively low concentrations, its ability to absorb IR makes it a major contributor to the greenhouse effect.
Water Vapour (H₂O)
Water vapour contains O–H bonds, which absorb IR radiation very effectively over a broad range of wavelengths. This makes water vapour the most significant natural greenhouse gas.
O–H stretching vibrations absorb strongly in the IR region.
The broad absorption range means more outgoing IR radiation is trapped.
Methane and Hydrocarbons
Methane and other hydrocarbons contain multiple C–H bonds. While individual C–H bonds absorb less strongly than O–H or C=O bonds, methane’s molecular structure allows absorption at wavelengths where Earth emits IR radiation efficiently.
The Greenhouse Effect
The greenhouse effect is the process by which certain gases absorb and re-emit IR radiation, warming the lower atmosphere.
Greenhouse effect: The warming of Earth’s atmosphere caused by absorption and re-emission of infrared radiation by atmospheric gases.
The process occurs in several stages:
Solar radiation reaches Earth, mainly as visible and ultraviolet light.
Earth’s surface absorbs energy and warms.
The warm surface emits energy as IR radiation.
Greenhouse gases absorb some of this outgoing IR radiation.
The absorbed energy is re-emitted in all directions, including back towards Earth.
Greenhouse gases absorb some of the outgoing infrared radiation and re-emit it in all directions, reducing the net loss of energy to space.
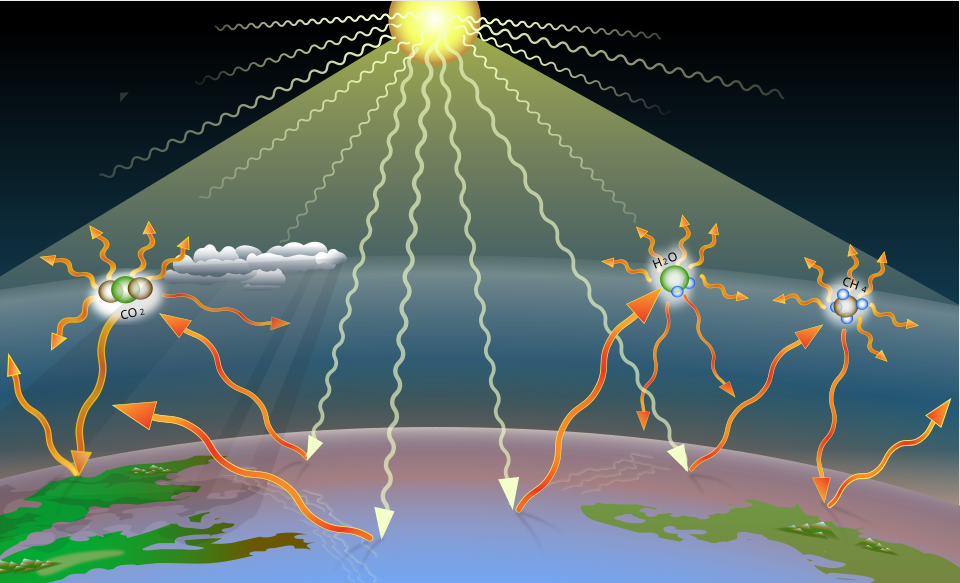
This diagram shows how incoming solar radiation is absorbed by Earth’s surface and re-emitted as infrared radiation. Greenhouse gases absorb some of this IR and re-emit it, including back towards the surface, increasing atmospheric warming. Source
This reduces the rate at which Earth loses energy to space, leading to an increase in average global temperatures.
Link to Global Warming
Global warming refers to the long-term increase in Earth’s average surface temperature. Enhanced greenhouse gas concentrations increase the amount of IR radiation absorbed in the atmosphere.
Global warming: The long-term rise in average global temperatures due to increased absorption of infrared radiation by greenhouse gases.
Human activities, particularly the combustion of fossil fuels, increase atmospheric concentrations of gases containing C=O, O–H, and C–H bonds:
Burning fossil fuels increases CO₂ levels.
Agriculture and waste processes increase methane emissions.
Industrial activity can alter water vapour distribution indirectly through warming.
As concentrations of these gases rise, more IR radiation is absorbed, intensifying the greenhouse effect.
Carbon dioxide has a particularly important absorption band in the longwave infrared around 15 μm, overlapping with wavelengths emitted strongly by Earth.
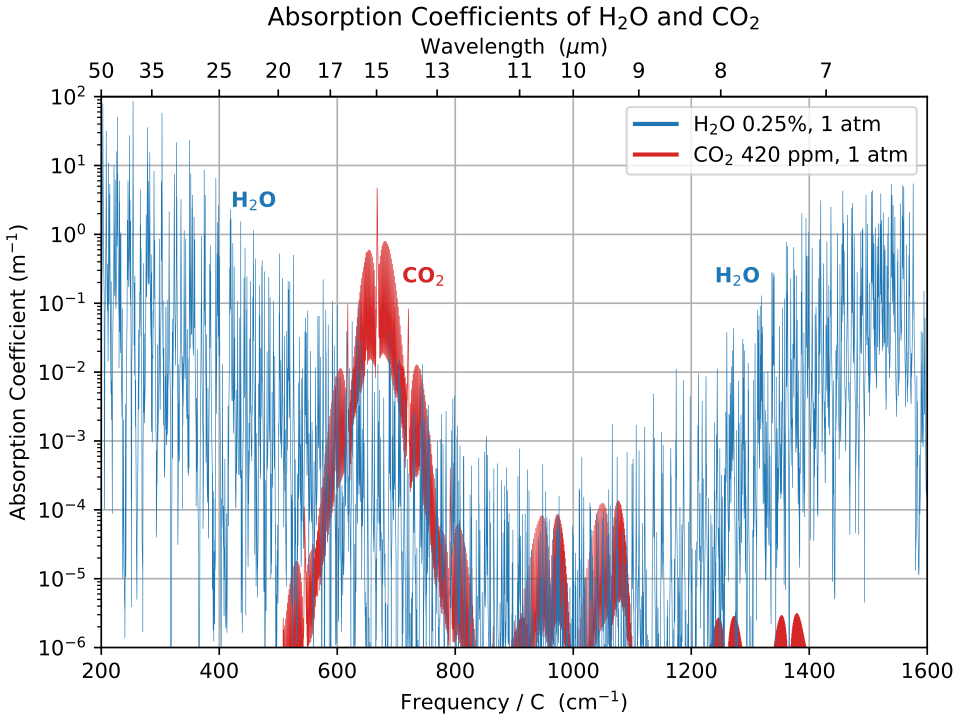
The graph shows how water vapour and carbon dioxide absorb longwave infrared radiation at different wavelengths. The strong CO₂ absorption band near 15 μm coincides with wavelengths at which Earth emits significant infrared energy. Source
Scientific Evidence and Energy-Use Policies
Understanding IR absorption by atmospheric gases provides the scientific basis for climate models and environmental policy decisions. IR spectroscopy allows scientists to:
Identify which gases absorb IR radiation.
Measure atmospheric concentrations of greenhouse gases.
Predict how changes in gas concentrations affect global temperatures.
Different greenhouse gases absorb infrared radiation in different wavelength ranges, so the atmosphere is not equally ‘opaque’ to IR at all wavelengths.
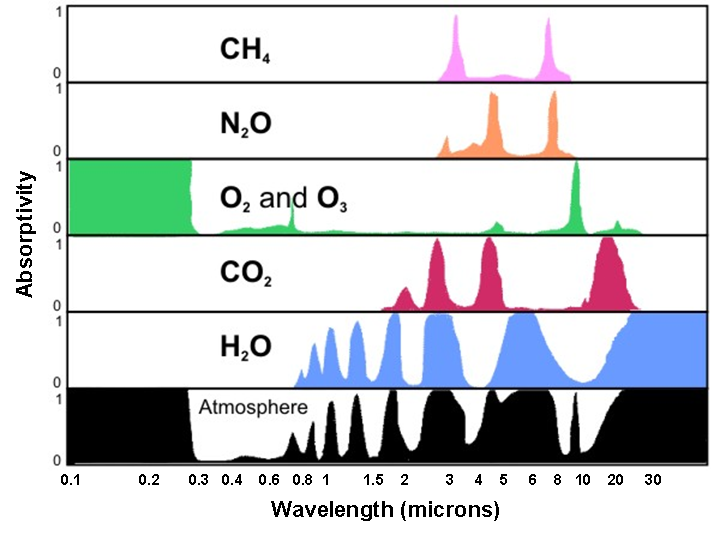
This figure compares infrared absorption by several atmospheric gases across wavelength. It includes gases beyond the OCR Chemistry focus, but clearly shows that absorption occurs in specific bands rather than uniformly across the spectrum. Source
This evidence informs energy-use policies aimed at reducing emissions, such as:
Limiting fossil fuel combustion.
Promoting renewable energy sources.
Improving energy efficiency to reduce CO₂ output.
The connection between molecular bond vibrations, IR absorption, and atmospheric warming demonstrates how fundamental chemical principles directly inform global environmental decision-making.
FAQ
For a gas to absorb infrared radiation, its molecules must experience a change in dipole moment when they vibrate.
Gases such as nitrogen (N₂) and oxygen (O₂) consist of identical atoms bonded together, so their bonds are non-polar. Vibrations in these molecules do not produce a changing dipole moment, meaning they are IR-inactive and do not contribute directly to the greenhouse effect.
Carbon dioxide absorbs infrared radiation at specific wavelengths that are not strongly absorbed by water vapour.
In particular, CO₂ absorbs IR near 15 μm, where Earth emits a large amount of infrared radiation. This means small increases in CO₂ concentration can significantly affect how much energy escapes to space, making CO₂ a key driver of long-term warming.
Molecular shape determines whether vibrations lead to a changing dipole moment.
For example, although CO₂ is a linear molecule overall, certain stretching vibrations make the molecule temporarily asymmetric. This allows IR absorption even though the molecule has no permanent dipole moment, highlighting the importance of vibrational motion rather than static structure.
Molecules can only vibrate at specific energies determined by their bond strengths and atomic masses.
As a result, each type of bond absorbs infrared radiation only at particular wavelength ranges, known as absorption bands. This explains why the atmosphere absorbs IR selectively rather than uniformly, allowing some infrared radiation to escape directly to space.
Infrared spectroscopy allows scientists to measure which wavelengths of IR radiation are absorbed by different gases.
By comparing these absorption patterns with the wavelengths of IR emitted by Earth, scientists can identify which gases reduce energy loss to space. This direct molecular evidence supports the link between greenhouse gas concentrations and global warming.
Practice Questions
Explain why carbon dioxide is able to absorb infrared radiation in the atmosphere.
(2 marks)
Award marks for:
Carbon dioxide contains polar C=O bonds that can absorb infrared radiation. (1 mark)
Absorption occurs because IR radiation increases vibrational motion of these bonds or causes a change in dipole moment. (1 mark)
Describe how absorption of infrared radiation by atmospheric gases contributes to global warming.
In your answer, refer to bond types and the movement of energy.
(5 marks)
Award marks for the following points (maximum 5 marks):
Earth absorbs energy and re-emits it as infrared radiation. (1 mark)
Atmospheric gases such as CO₂ and H₂O contain C=O or O–H bonds that absorb IR radiation. (1 mark)
Absorption of IR increases vibrational energy within these molecules. (1 mark)
Absorbed energy is re-emitted in all directions, including back towards Earth’s surface. (1 mark)
This reduces energy loss to space, causing an increase in average global temperature (global warming). (1 mark)
Do not award marks for vague references to “heat being trapped” without reference to infrared radiation or molecular bonding.

