OCR Specification focus:
‘Use mass spectra to find the molecular ion peak (molecular mass) and analyse fragmentation patterns to identify parts of structures; note small M+1 from 13C.’
Mass spectrometry is a powerful analytical technique used in organic chemistry to determine molecular mass and structural features by analysing ionised fragments of compounds.
Mass Spectrometry: Purpose and Overview
Mass spectrometry is used to identify compounds and deduce structural information by measuring the mass-to-charge ratio (m/z) of ions produced from a sample. In OCR A-Level Chemistry, emphasis is placed on recognising the molecular ion peak, interpreting fragmentation patterns, and understanding the significance of the M+1 peak arising from carbon-13.
Mass spectrometry: An analytical technique that ionises chemical substances and separates the resulting ions according to their mass-to-charge ratio.
A mass spectrum is produced as a graph of relative abundance against m/z, showing how many ions of each mass are detected.
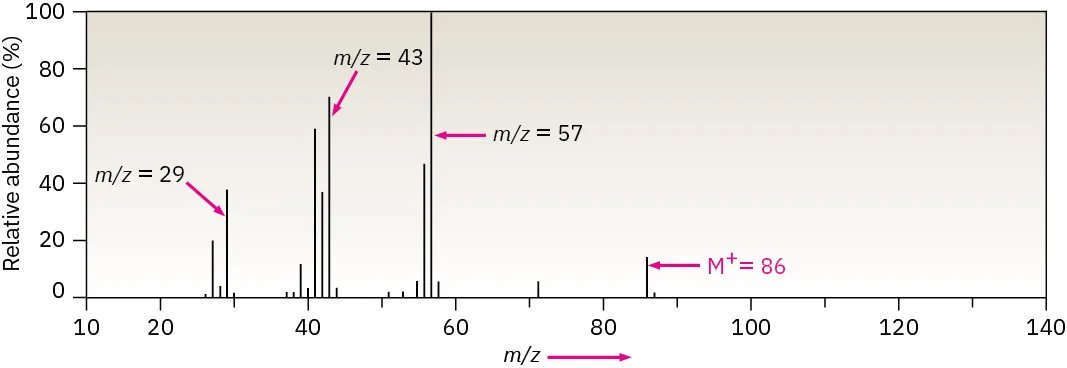
Mass spectrum of hexane showing fragment peaks at lower m/z values and the molecular ion peak (M⁺) at higher m/z. The labelled fragment ions illustrate how bond cleavage patterns provide structural information. Source
Ionisation and Formation of the Molecular Ion
In organic mass spectrometry, compounds are ionised by electron impact. High-energy electrons collide with gaseous molecules, removing an electron and forming a positively charged ion.
Electron impact ionisation
M(g) + e⁻ → M⁺(g) + 2e⁻
M = molecule in gaseous state
M⁺ = molecular ion
The molecular ion is crucial because it usually has the same mass as the original molecule, allowing determination of the relative molecular mass (Mr).
Molecular ion (M⁺): A positively charged ion formed when a molecule loses one electron without breaking apart.
Not all compounds produce a strong molecular ion peak; some fragment extensively, leading to a weak or absent M⁺ peak.
The Molecular Ion Peak (M⁺)
The molecular ion peak corresponds to the ion with the highest m/z value that is not due to isotopes. It provides direct information about the molecular mass of the compound.
Key points for identifying the molecular ion peak:
It appears at the highest significant m/z value
It represents the unfragmented ion
Its m/z value equals the relative molecular mass of the compound
It is labelled M⁺
For OCR purposes, students should be confident in using the M⁺ peak to determine molecular mass before analysing fragmentation data.
Fragmentation and Fragment Ions
After ionisation, the molecular ion may break apart into smaller pieces known as fragment ions. These fragments form due to bond cleavage within the molecular ion and provide valuable structural information.
Fragment ion: A positively charged ion formed when a molecular ion breaks into smaller pieces during mass spectrometry.
Fragmentation patterns depend on:
Bond strengths within the molecule
Stability of the resulting ions
Presence of functional groups
More stable carbocations are more likely to form, influencing which fragment peaks appear most prominently.
Interpreting Fragmentation Patterns
Fragmentation patterns are analysed by examining the m/z values of fragment peaks and considering which parts of the molecule could produce ions of those masses.
Important principles include:
Weaker bonds break more readily, leading to common fragment peaks
Alkyl groups often form stable carbocations
Certain functional groups produce characteristic fragments
The base peak is the tallest peak in the spectrum and represents the most abundant ion, not necessarily the molecular ion.
Base peak: The peak with the greatest intensity in a mass spectrum, assigned a relative abundance of 100%.
Although the base peak is often a fragment ion, it provides important clues about the most stable ion formed during fragmentation.
The M+1 Peak and Carbon-13
In addition to the molecular ion peak, a small peak often appears at one unit higher m/z, known as the M+1 peak. This arises due to the natural occurrence of the carbon-13 isotope.
Carbon exists naturally as:
¹²C, which is most abundant
¹³C, present at approximately 1.1%
Molecules containing carbon therefore have a small proportion with one ¹³C atom, producing ions with a mass one unit higher than the M⁺ peak.
Key points about the M+1 peak:
It is always smaller than the M⁺ peak
Its relative size increases with the number of carbon atoms
It confirms the presence of carbon in the compound
For OCR A-Level Chemistry, students are not required to calculate exact carbon numbers but should recognise the origin and significance of the M+1 peak.
Using Mass Spectra in Structural Analysis
Mass spectrometry is rarely used in isolation. However, within the syllabus, it is used to:
Determine molecular mass using the M⁺ peak
Identify likely fragments to infer structural features
Recognise isotopic patterns such as M+1
When combined with other analytical techniques later in the course, mass spectra contribute to confident identification of organic structures while reinforcing understanding of bonding and stability.
In a typical mass spectrometer, molecules are ionised, then the ions are separated according to m/z before being detected.
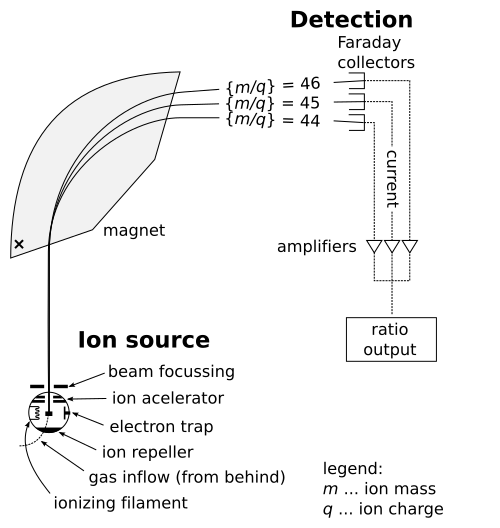
Schematic diagram of a mass spectrometer showing ion formation, separation by mass-to-charge ratio, and detection. The analyser region is simplified; real instruments may use different analyser designs. Source
Throughout interpretation, careful attention should be paid to peak positions, relative intensities, and logical fragmentation pathways consistent with known chemical behaviour.
FAQ
Some molecules fragment very easily after ionisation, so the molecular ion breaks apart before it can be detected.
This is common in:
Branched alkanes and alcohols
Molecules with weaker bonds or unstable molecular ions
As a result, fragment peaks dominate the spectrum and the M⁺ peak may be small or absent.
Fragment ions form as positively charged species, often carbocations, whose stability affects their likelihood of appearing.
More stable carbocations form more readily, such as:
Tertiary carbocations
Secondary carbocations over primary
This is why certain fragment peaks are more intense and can help indicate branching within a molecule.
The base peak represents the most abundant ion, not the heaviest one.
Lower m/z fragment ions are often more stable and formed in greater numbers, so they dominate the spectrum even though they represent only part of the original molecule.
No. The size of the M+1 peak depends on how many carbon atoms are present.
Molecules with more carbon atoms have a higher chance of containing one 13C atom, increasing the relative intensity of the M+1 peak.
Yes. Different compounds can share the same relative molecular mass and therefore have identical M⁺ values.
However, their fragmentation patterns are usually different, allowing the compounds to be distinguished by analysing their fragment peaks rather than relying solely on the molecular ion.
Practice Questions
A mass spectrum of an organic compound shows a molecular ion peak at m/z = 72 and a smaller peak at m/z = 73.
a) State what information can be obtained from the molecular ion peak at m/z = 72.
b) Explain the origin of the peak at m/z = 73.
(2 marks)
a)
States that the molecular ion peak gives the relative molecular mass of the compound (1 mark)
b)
Identifies the peak as the M+1 peak (1 mark)
Explains that it is due to the presence of carbon-13 atoms in some molecules (1 mark)
(Maximum 2 marks; explanation must link 13C to increased mass)
An organic compound produces the following features in its mass spectrum:
A molecular ion peak (M⁺) at m/z = 86
A base peak at m/z = 43
Several fragment peaks at lower m/z values
a) Explain what is meant by the term molecular ion.
b) Describe how fragmentation occurs in a mass spectrometer.
c) Explain why the base peak does not necessarily correspond to the molecular ion.
(5 marks)
a)
Defines molecular ion as a positively charged ion formed by loss of one electron without fragmentation (1 mark)
b)
States that molecules are ionised by electron impact (1 mark)
Explains that excess energy causes bonds to break, forming fragment ions (1 mark)
c)
Identifies the base peak as the most abundant ion (1 mark)
Explains that it corresponds to the most stable fragment ion, not necessarily the molecular ion (1 mark)
(Maximum 5 marks; answers must be clearly linked to mass spectrometry processes)

