OCR Specification focus:
‘Infrared radiation increases covalent bond vibrations; identify characteristic absorptions for O–H and C=O; most organic compounds show an absorption near 3000 cm–1 from C–H.’
Infrared spectroscopy is a core analytical technique that links molecular bond vibrations to characteristic absorption peaks, allowing chemists to identify functional groups reliably in practice.
Fundamentals of Infrared Spectroscopy
Infrared (IR) spectroscopy is based on the interaction between infrared radiation and molecules. When IR radiation passes through a sample, specific frequencies are absorbed, causing covalent bonds within molecules to vibrate. The pattern of absorbed frequencies produces an infrared spectrum, which provides information about the bonds present in a compound.
Infrared radiation: Electromagnetic radiation with energies lower than visible light that causes covalent bonds in molecules to vibrate.
Only bonds that involve a change in dipole moment during vibration absorb infrared radiation. This is why IR spectroscopy is particularly effective for identifying polar bonds such as O–H and C=O.
Heading 2
Bond Vibrations and Absorption
Covalent bonds behave like tiny springs, vibrating continuously. When the frequency of incoming IR radiation matches the natural vibrational frequency of a bond, absorption occurs. Different bonds absorb at different frequencies because vibrational energy depends on:
The bond strength (bond enthalpy)
The masses of the bonded atoms
The bond polarity
Stronger bonds vibrate at higher frequencies and therefore absorb radiation at higher wavenumbers. For example, a double bond absorbs at a higher wavenumber than a single bond between the same atoms.
Wavenumber: A measure of infrared radiation frequency, defined as the number of waves per centimetre (cm⁻¹).
Wavenumber is used rather than wavelength because it is directly proportional to energy, making comparisons between absorption peaks clearer.
Types of Bond Vibrations
Bonds can vibrate in several ways, but OCR A-Level Chemistry focuses on stretching vibrations, which are most relevant for functional group identification.
Stretching vibrations involve changes in bond length and include:
Symmetrical stretching, where bonds stretch together
Asymmetrical stretching, where bonds stretch unevenly
Bending vibrations also exist but are less emphasised at this level. Stretching vibrations produce clearer, more diagnostic absorption peaks.
There must be a net change in dipole moment during vibration for absorption to occur. Non-polar bonds such as O=O do not absorb IR radiation and are therefore invisible in IR spectra.
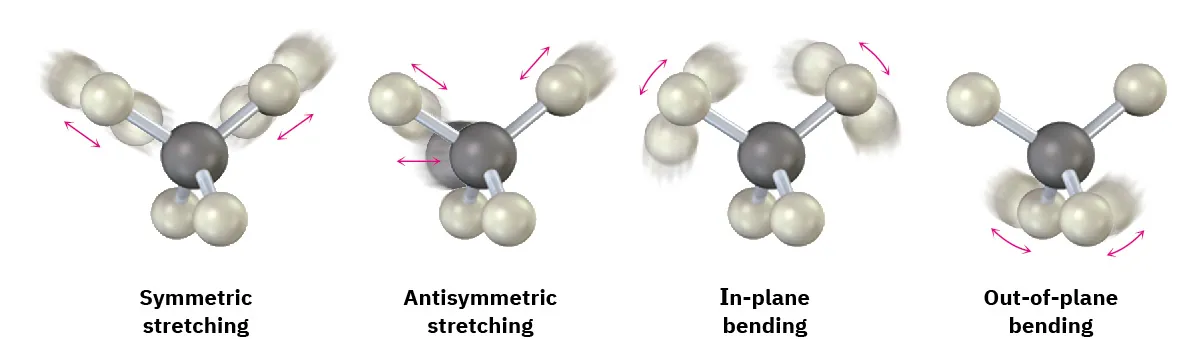
This diagram illustrates common bond vibration modes detected by IR spectroscopy, including stretching and bending. Different vibration types absorb infrared radiation at different wavenumbers, producing characteristic peaks. Extra detail includes in-plane and out-of-plane bending, which extends beyond the OCR minimum but aids conceptual understanding. Source
Characteristic Absorption Peaks
Each functional group produces absorption peaks in characteristic regions of the infrared spectrum. These regions are consistent and allow functional groups to be identified reliably using provided data.
O–H Absorption (Alcohols)
The O–H bond is highly polar and absorbs strongly in IR spectroscopy. Alcohols show a distinctive absorption due to O–H stretching.
Key features of the O–H absorption:
Appears as a broad peak
Found between 3200–3600 cm⁻¹
Broadness caused by hydrogen bonding between molecules
Hydrogen bonding results in a range of bond strengths, spreading absorption over many frequencies and producing the characteristic broad shape.
C=O Absorption (Carbonyl Compounds)
The carbonyl group (C=O) produces one of the most intense and reliable absorptions in IR spectroscopy.
Important characteristics include:
A strong, sharp peak
Located around 1700 cm⁻¹
Caused by stretching of the polar C=O double bond
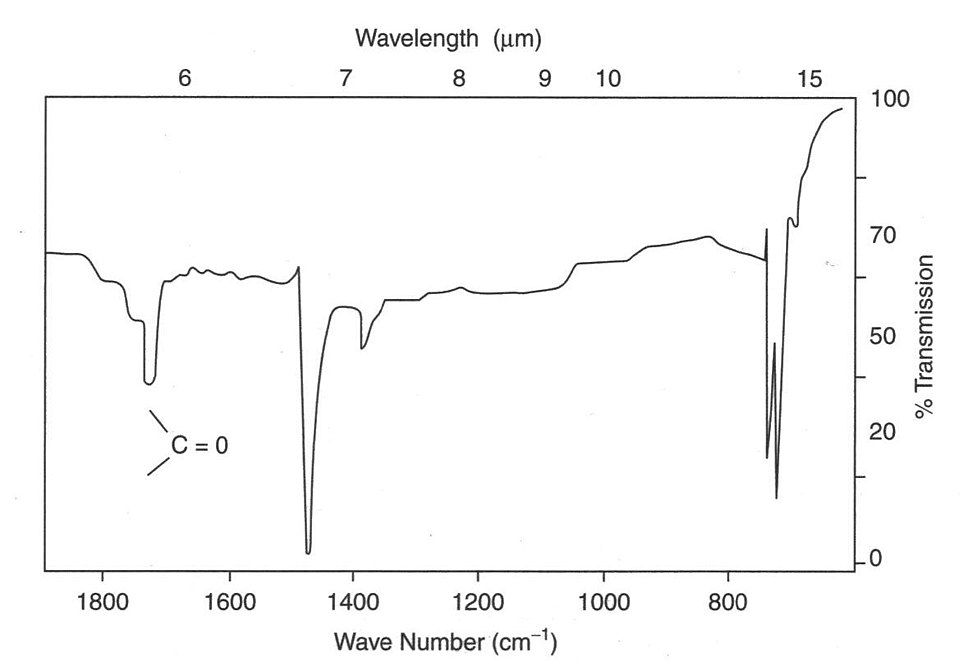
This infrared spectrum highlights a strong absorption attributed to C=O stretching near 1700 cm⁻¹. The pronounced depth of the peak reflects strong absorption by the polar carbon–oxygen double bond. Extra detail includes additional labelled regions not required by the OCR specification. Source
This absorption is present in aldehydes, ketones, carboxylic acids, and esters, making it a key indicator of carbonyl-containing compounds.
C–H Absorption Near 3000 cm⁻¹
Most organic compounds contain C–H bonds, and these produce absorptions near 3000 cm⁻¹, as specified by OCR.
Features of C–H absorption:
Appears as multiple small peaks
Typically between 2850–3000 cm⁻¹
Present in alkanes, alcohols, aldehydes, ketones, and acids
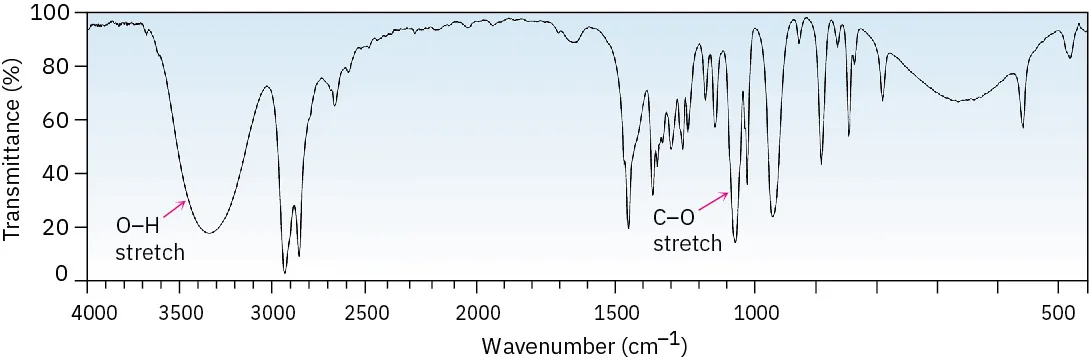
This infrared spectrum shows the broad O–H stretching absorption between 3200–3600 cm⁻¹, characteristic of alcohols with hydrogen bonding. Peaks near 3000 cm⁻¹ correspond to C–H stretching found in most organic compounds. Extra detail includes a labelled C–O stretch, which is not required for OCR 8.4.1. Source
Because C–H bonds are so common, this absorption confirms the presence of an organic compound but does not distinguish between different functional groups.
Interpreting an Infrared Spectrum
An IR spectrum plots percentage transmittance against wavenumber. Absorption peaks appear as downward dips, where radiation has been absorbed by the sample.
When analysing a spectrum:
Identify strong, diagnostic peaks first
Look for O–H, C=O, and C–H absorptions
Ignore minor peaks unless relevant to known functional groups
The region below 1500 cm⁻¹ is often called the fingerprint region. While unique to each compound, it is not used for detailed interpretation at OCR A-Level.
Heading 2
Importance of IR Spectroscopy in Organic Analysis
IR spectroscopy is a rapid and non-destructive technique used to confirm the presence or absence of key functional groups. It does not give full structural information but is essential for narrowing down possibilities when analysing unknown organic compounds.
In OCR A-Level Chemistry, students are expected to:
Recognise characteristic O–H and C=O absorptions
Identify the C–H absorption near 3000 cm⁻¹
Understand how bond vibrations relate to absorption peaks
Mastery of these principles allows confident interpretation of provided IR data in assessments.
FAQ
Only bonds that experience a change in dipole moment during vibration can absorb infrared radiation. This means the bond must be polar or become temporarily polar as it vibrates.
Non-polar bonds, such as O=O or N≡N, do not produce an absorption because there is no change in charge distribution during vibration. This is why IR spectroscopy is particularly effective for identifying polar functional groups like O–H and C=O.
The fingerprint region refers to the area below about 1500 cm⁻¹ in an infrared spectrum. It contains many closely spaced absorption peaks caused by complex bending and stretching vibrations.
Although this region is unique to each compound, it is not used for functional group identification at OCR A-Level. Instead, it is mainly used to compare an unknown spectrum with a known reference spectrum.
Peak intensity depends on how much the dipole moment changes during vibration.
Bonds such as C=O and O–H are highly polar, so their vibrations cause large changes in dipole moment, resulting in strong absorptions. Less polar bonds, such as C–H, produce weaker peaks even though they are very common in organic compounds.
Stronger bonds require more energy to vibrate. As a result, they absorb infrared radiation at higher wavenumbers.
For example, a C=O double bond absorbs at a higher wavenumber than a C–O single bond. This relationship allows chemists to distinguish between different types of covalent bonds using IR spectra.
Wavenumber is directly proportional to the energy of infrared radiation, making it easier to compare absorption energies.
Using cm⁻¹ also spreads the infrared spectrum out more evenly, allowing characteristic absorptions for different bonds to appear in consistent, easily recognised regions across spectra.
Practice Questions
Infrared spectroscopy is used to analyse an organic compound that contains only carbon, hydrogen and oxygen.
State the wavenumber region you would expect to see an absorption due to an O–H bond and explain why this absorption is broad.
(2 marks)
States correct O–H absorption region: 3200–3600 cm⁻¹ (1 mark)
Explains broad peak due to hydrogen bonding between molecules causing a range of O–H bond strengths or vibrational energies (1 mark)
An infrared spectrum of an unknown organic compound shows the following features:
A strong absorption at approximately 1700 cm⁻¹
A broad absorption between 3200 and 3600 cm⁻¹
Several absorptions near 3000 cm⁻¹
(a) Identify two different functional groups present in the compound using this data.
(b) Explain why absorptions are seen at approximately 1700 cm⁻¹ and near 3000 cm⁻¹, referring to bond vibrations and bond polarity.
(5 marks)
(a) Identification of functional groups (2 marks)
Identifies presence of an alcohol from broad O–H absorption at 3200–3600 cm⁻¹ (1 mark)
Identifies presence of a carbonyl group (aldehyde, ketone, carboxylic acid, or ester) from strong absorption near 1700 cm⁻¹ (1 mark)
(b) Explanation of absorptions (3 marks)
Explains that the absorption at ~1700 cm⁻¹ is due to stretching of a polar C=O double bond (1 mark)
Explains that absorptions near 3000 cm⁻¹ are due to C–H stretching vibrations (1 mark)
Links absorption to infrared radiation matching the natural vibrational frequency of the bond or to changes in dipole moment during vibration (1 mark)

