OCR Specification focus:
‘Use IR spectra to identify alcohols (O–H), aldehydes/ketones (C=O), and carboxylic acids (broad O–H plus C=O); data will be provided in exams.’
Infrared spectroscopy allows chemists to identify functional groups by analysing characteristic bond absorptions, providing rapid, non-destructive evidence for key organic structures.
Purpose of IR Spectroscopy in Functional Group Identification
Infrared (IR) spectroscopy is an analytical technique used to determine which functional groups are present in an organic compound. At OCR A-Level, IR is not used to determine full molecular structures but to confirm or exclude specific functional groups listed in the specification.
IR spectra show absorption peaks caused by bond vibrations within molecules. Different bonds absorb IR radiation at characteristic wavenumbers, allowing chemists to match peaks to known functional groups using provided data.
Fundamental Principle of IR Absorption
Infrared spectroscopy: An analytical technique that measures absorption of infrared radiation by covalent bonds, causing vibrational transitions characteristic of specific bonds.
IR radiation causes bonds to vibrate by stretching or bending. Absorption occurs only when the vibration causes a change in dipole moment, which is why some bonds give strong absorptions while others are weak or absent.
The horizontal axis of an IR spectrum is wavenumber (cm⁻¹), decreasing from left to right. The vertical axis shows percentage transmittance or absorption intensity.
Key Functional Groups in the OCR Specification
Only functional groups specified by OCR should be identified from IR spectra. Students are expected to recognise patterns rather than memorise entire spectra.
Alcohols: O–H Absorption
Alcohols contain the hydroxyl (–OH) functional group, which produces a highly recognisable absorption.
Key features for alcohols:
Broad O–H stretch between 3200–3600 cm⁻¹
Caused by extensive hydrogen bonding between molecules
Often overlaps other peaks but remains distinguishable due to its width
An alcohol is identified in an IR spectrum by a broad O–H stretch in the 3200–3600 cm–1 region.
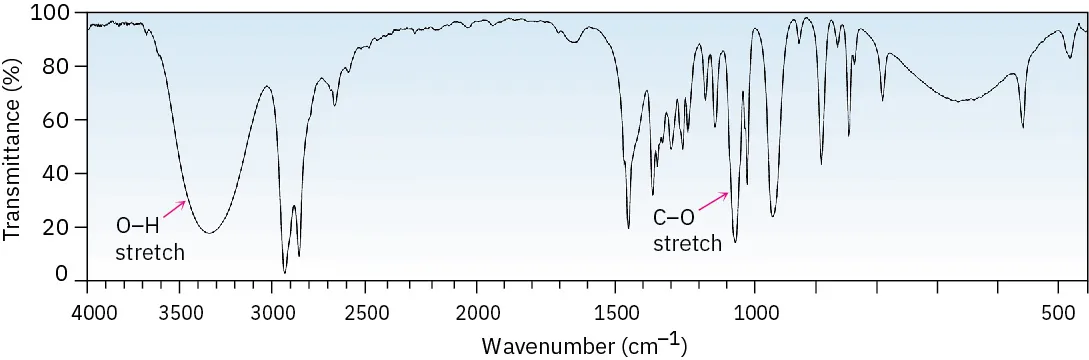
This IR spectrum (cyclohexanol) highlights the broad O–H stretching absorption caused by hydrogen bonding. The labelled C–O stretch sits in the fingerprint region and supports the alcohol assignment alongside the O–H band. Source
The broad nature of the peak is a crucial diagnostic feature. A sharp peak in this region would suggest a different functional group, not an alcohol.
Alcohols do not contain a C=O bond, so no strong absorption appears around 1700 cm⁻¹.
Aldehydes and Ketones: C=O Absorption
Both aldehydes and ketones contain the carbonyl (C=O) functional group, which gives a strong and sharp absorption.
Key features for aldehydes and ketones:
Strong, sharp C=O stretch around 1650–1750 cm⁻¹
One of the most intense peaks in most organic IR spectra
Indicates presence of a carbonyl-containing compound
A C=O (carbonyl) stretch appears as a strong absorption near 1700 cm–1, so its presence strongly suggests an aldehyde, ketone, or carboxylic acid functional group.
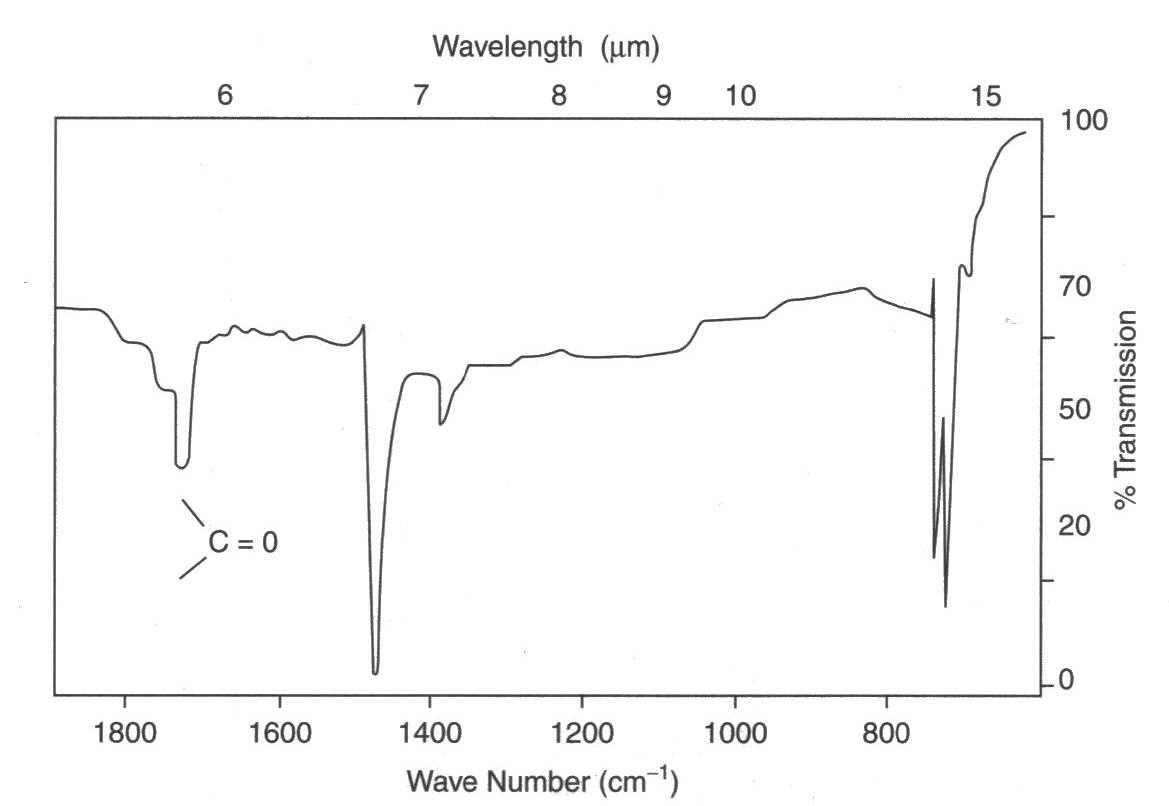
This spectrum highlights a labelled C=O absorption in the ~1700 cm–1 region, illustrating the diagnostic carbonyl peak shape and position. Extra detail not required by the syllabus: the sample is photodegraded polyethylene, and the axes use German terms. Source
IR spectroscopy cannot distinguish between aldehydes and ketones on its own because both contain the same C=O bond. Further tests or data are required for differentiation.
Students should focus on recognising the presence of the carbonyl group rather than attempting detailed structural interpretation.
Carboxylic Acids: Combined O–H and C=O Features
Carboxylic acids contain both a carbonyl (C=O) and a hydroxyl (O–H) group, producing a distinctive combination of absorptions.
Key features for carboxylic acids:
Very broad O–H stretch from approximately 2500–3300 cm⁻¹
Strong C=O stretch around 1700 cm⁻¹
O–H peak broader and lower than in alcohols due to strong hydrogen bonding
A carboxylic acid shows a very broad O–H absorption between 2500–3300 cm–1 and a strong C=O absorption around 1700 cm–1.
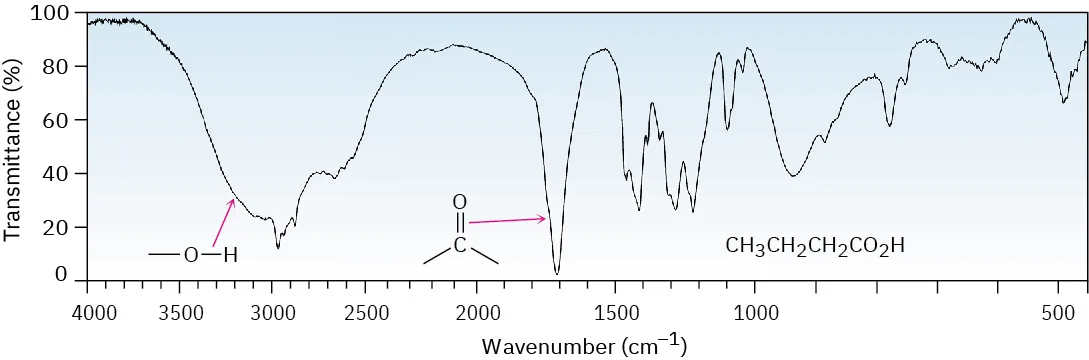
This IR spectrum (butanoic acid) shows the very broad O–H absorption extending across the 2500–3300 cm–1 region and the strong C=O peak around ~1700 cm–1. Seeing both together is the key pattern for identifying a carboxylic acid rather than an alcohol. Source
The extremely broad O–H absorption is the most diagnostic feature and often extends into the C–H region, making it easy to recognise.
Common Absorptions Seen in Most Organic Molecules
In addition to functional group peaks, most organic compounds show absorptions that are not diagnostic of specific functional groups but help confirm organic nature.
Common features include:
C–H stretch near 2850–3000 cm⁻¹
Present in alkanes, alcohols, aldehydes, ketones, and carboxylic acids
Not used to distinguish functional groups at OCR level
These peaks should be recognised but not overinterpreted.
Using Provided Data in OCR Examinations
OCR examinations will always provide relevant IR absorption data. Students are not required to memorise exact wavenumbers but should be able to:
Match observed peaks to supplied data
Identify whether key functional groups are present or absent
Use absence of peaks as evidence to eliminate possibilities
When analysing a spectrum:
Look first for a C=O peak near 1700 cm⁻¹
Then check for broad O–H absorptions
Use combinations of peaks rather than isolated absorptions
Limitations of IR Spectroscopy at A-Level
IR spectroscopy has clear limitations that students must understand.
Key limitations:
Cannot identify the full molecular structure
Cannot distinguish between aldehydes and ketones
Cannot identify position of functional groups within a molecule
Only functional groups in the specification should be considered
IR spectroscopy is therefore best used alongside other analytical techniques such as mass spectrometry, but this is handled in a separate subtopic.
Exam-Focused Interpretation Skills
At OCR A-Level, success depends on recognising patterns, not analysing spectra in excessive detail.
Students should practise:
Identifying broad versus sharp peaks
Recognising strong absorptions
Using logical elimination based on missing peaks
Linking spectra directly to specification functional groups
Correct interpretation relies on careful observation and strict adherence to the functional groups defined by the specification, ensuring accurate and efficient analysis under exam conditions.
FAQ
The O–H absorption in carboxylic acids is broader because carboxylic acid molecules form strong hydrogen-bonded dimers.
These dimers create a wide range of O–H bond environments, leading to absorption over a much wider wavenumber range than in alcohols.
In alcohols, hydrogen bonding is weaker and more variable, so the O–H peak is broad but noticeably narrower than that seen in carboxylic acids.
The C=O bond is highly polar due to the large electronegativity difference between carbon and oxygen.
When the C=O bond stretches, there is a significant change in dipole moment, causing strong absorption of infrared radiation.
This makes the carbonyl peak one of the most intense and easily recognised absorptions in organic IR spectra.
Both aldehydes and ketones contain the same carbonyl (C=O) functional group.
As a result, they produce very similar C=O stretching absorptions at around 1700 cm–1.
At OCR A-Level, subtle differences in peak position are not required knowledge, so additional chemical tests or analytical data are needed to tell them apart.
The position of the C=O absorption can shift slightly depending on the surrounding molecular structure.
Factors include:
adjacent functional groups
degree of electron donation or withdrawal
hydrogen bonding interactions
However, these shifts are small and students should always rely on provided data rather than memorising exact values.
The fingerprint region contains many overlapping absorptions from complex bond vibrations.
These patterns are unique to individual molecules rather than functional groups, making them difficult to interpret reliably.
OCR A-Level questions focus only on clear, diagnostic absorptions linked directly to functional groups listed in the specification.
Practice Questions
An infrared (IR) spectrum of an organic compound shows a broad absorption between 2500 and 3300 cm–1 and a strong sharp absorption at about 1700 cm–1.
Identify the functional group present and give one reason for your answer.
(2 marks)
Identifies the functional group as a carboxylic acid (1 mark)
Gives a correct reason:
very broad O–H absorption at 2500–3300 cm–1 or
presence of a C=O absorption near 1700 cm–1 or
mentions both O–H and C=O together (1 mark)
An organic compound has the molecular formula C3H6O2. Its infrared (IR) spectrum shows:
a very broad absorption between 2500 and 3300 cm–1
a strong absorption at approximately 1700 cm–1
an absorption near 3000 cm–1
Use this information to:
a) Identify the functional group present in the compound.
b) Explain how the IR spectrum supports your identification.
c) State one absorption in the spectrum that does not help to distinguish between functional groups.
(5 marks)
a) Identification (1 mark)
Carboxylic acid
b) Explanation using IR data (3 marks)
Very broad O–H absorption between 2500–3300 cm–1 due to hydrogen bonding in carboxylic acids (1 mark)
Strong sharp C=O absorption around 1700 cm–1 indicating a carbonyl group (1 mark)
Combination of broad O–H and C=O absorptions is characteristic of carboxylic acids (1 mark)
c) Non-diagnostic absorption (1 mark)
Absorption near 3000 cm–1 due to C–H stretching or
States that the C–H absorption is present in most organic compounds and does not distinguish functional groups

