OCR Specification focus:
‘For multi-step reactions, link the rate-determining step to the rate equation and propose consistent mechanism steps.’
These notes explain how multi-step reaction mechanisms control observed rates, showing how the slowest step determines rate equations and constrains plausible mechanistic pathways in chemistry.
Multi-step reactions and mechanisms
Many chemical reactions occur through multiple elementary steps rather than a single collision. Each step represents an individual molecular event with its own rate and molecularity. The full sequence of steps that describes how reactants form products is known as the reaction mechanism.
Mechanism: A sequence of elementary steps that together describe the molecular-level pathway by which a chemical reaction occurs.
Between reactants and products, mechanisms often involve intermediates, which are species formed in one step and consumed in a later step. These intermediates do not appear in the overall equation. A valid mechanism must:
Add up to the overall balanced equation.
Contain only feasible elementary steps.
Be consistent with experimentally observed rate data.
Understanding how the rate relates to individual steps is essential for linking mechanisms to kinetics.
The rate-determining step
In a multi-step mechanism, not all steps occur at the same speed. One step is usually significantly slower than the others, limiting how fast the overall reaction can proceed.
Rate-determining step: The slowest step in a multi-step reaction mechanism that controls the overall rate of the reaction.
Because faster steps can only occur as quickly as the slow step supplies reactants or intermediates, the overall reaction rate is governed by this rate-determining step (RDS). This idea is analogous to a bottleneck restricting flow in a system.
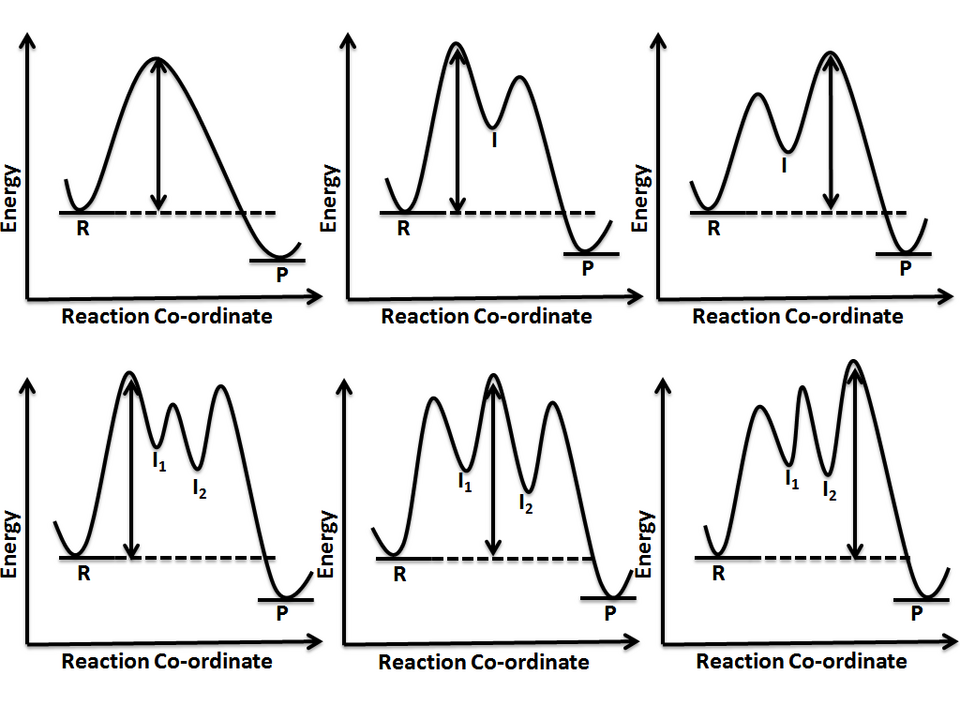
These reaction coordinate diagrams show how multi-step mechanisms contain multiple transition states (peaks) and sometimes intermediates (valleys). The step with the highest peak corresponds to the rate-determining step that limits the overall reaction rate. Extra detail: the figure also compares mechanisms with different numbers of intermediates. Source
The identification of the rate-determining step allows chemists to connect observed kinetic data with a plausible sequence of molecular events.
Linking the rate-determining step to the rate equation
The OCR specification requires that the rate equation is linked directly to the rate-determining step. The key principle is that the rate equation depends on the reactants involved in the slow step only.
Rate equation (general) = k × [reactant₁]ᵐ[reactant₂]ⁿ
k = rate constant (units depend on overall order)
[reactant] = concentration of reactant in the rate-determining step (mol dm⁻³)
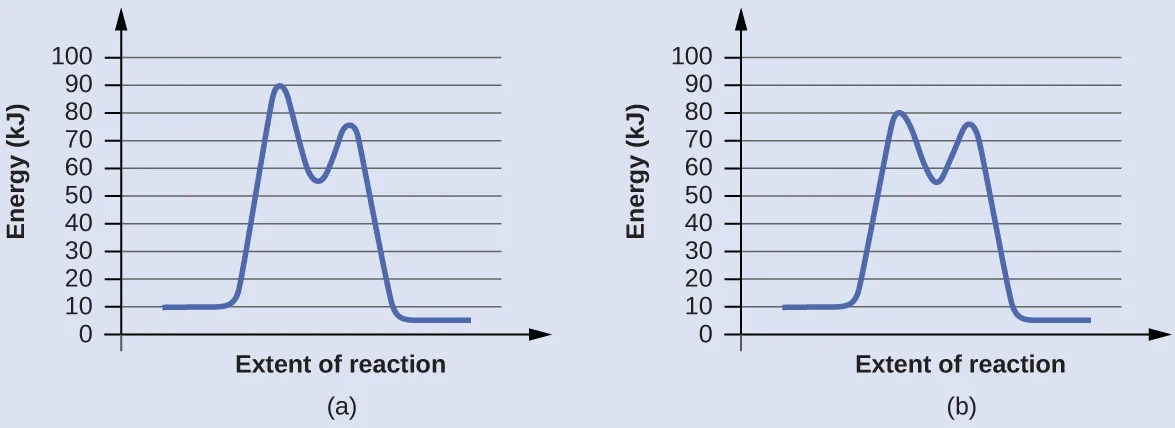
These energy profiles illustrate a two-step reaction in which the first step has the higher activation energy and is therefore rate-determining. The height of the highest peak identifies the slow step that controls the overall reaction rate. Extra detail: the surrounding text discusses catalysis, which is not required here. Source
For an elementary step, the powers in the rate equation correspond to the stoichiometric coefficients of reactants in that step. This is because elementary steps represent single molecular events, not averaged processes.
A rate equation derived from the RDS must:
Match the experimentally determined orders.
Contain only species present before or during the slow step.
Exclude products formed after the RDS.
If a proposed mechanism predicts a rate equation that disagrees with experimental data, the mechanism must be rejected or modified.
Role of intermediates in mechanisms
Intermediates are central to understanding mechanisms but must be treated carefully when considering rate equations. Although intermediates may appear in the slow step, they are often produced in an earlier fast step.
Because intermediates are not present at the start of the reaction, they must not appear in the final rate equation. Acceptable mechanisms ensure that:
Intermediates cancel out when steps are combined.
The rate equation can be expressed only in terms of reactants whose concentrations can be controlled or measured.
This requirement places strong constraints on which mechanisms are chemically reasonable.
Proposing consistent mechanism steps
The specification also requires proposing mechanism steps consistent with the rate equation. A valid proposed mechanism must align with both kinetic evidence and chemical logic.
When proposing a mechanism:
Begin with the overall balanced equation.
Introduce intermediates only when chemically justified.
Identify one clear rate-determining step.
Ensure fast steps do not contradict the observed rate law.
Steps should be labelled as fast or slow, reflecting their relative speeds. Only the slow step is used to deduce the rate equation, but the fast steps must still be chemically plausible and reversible where appropriate.
Importance of the rate-determining step concept
The concept of the rate-determining step explains why changes in concentration affect reaction rates in specific ways. It also clarifies why catalysts work by providing an alternative mechanism with a different, lower-energy slow step.
By linking the rate equation directly to the slowest step, chemists can:
Infer molecular-level details from kinetic experiments.
Test proposed mechanisms against experimental data.
Understand how altering conditions or catalysts changes reaction behaviour.
This connection between kinetics and mechanism is a core requirement of OCR A-Level Chemistry and underpins much of modern chemical reaction analysis.
FAQ
The overall equation shows only the starting materials and final products, not how the reaction occurs.
In multi-step reactions, the rate depends on the slowest elementary step, which may involve fewer or different species than the overall equation suggests.
Because of this, stoichiometric coefficients in the overall equation do not reliably indicate reaction order unless the reaction is known to be a single-step process.
A proposed mechanism must produce a rate equation that matches experimental data.
If the mechanism predicts:
Different reaction orders from those observed
A rate equation containing intermediates
Dependence on a species shown experimentally to have no effect
then the mechanism is inconsistent with the evidence and must be rejected or revised.
At A-Level, reactions are treated as having one clearly defined rate-determining step.
In reality, two steps can have similar rates, but this adds complexity beyond the OCR syllabus.
For exam purposes, the step labelled “slow” is always assumed to be much slower than the others and therefore controls the overall rate.
Fast steps explain how intermediates are formed and consumed and ensure the mechanism is chemically realistic.
They must:
Combine correctly to give the overall equation
Involve feasible collisions or bond changes
Avoid contradicting the experimentally determined rate equation
A mechanism with a correct slow step but unrealistic fast steps is still invalid.
Knowing the rate-determining step shows which molecular event limits the reaction speed.
This helps chemists:
Decide which reactant concentrations will most affect rate
Design catalysts that lower the activation energy of the slow step
Modify conditions to speed up industrial or laboratory reactions
This approach links kinetic data directly to practical chemical control.
Practice Questions
A reaction proceeds via the following two-step mechanism:
Step 1: A + B → C (slow)
Step 2: C + D → E (fast)
(a) Identify the rate-determining step.
(b) Write the rate equation for the overall reaction.
(2 marks)
(a)
Correct identification of Step 1 as the rate-determining step (1 mark)
(b)
Correct rate equation: rate = k[A][B] (1 mark)
A chemical reaction is found experimentally to have the rate equation:
rate = k[X][Y]
A student proposes the following mechanism:
Step 1: X + Y → Z (slow)
Step 2: Z + Y → P (fast)
(a) Explain why Step 1 is the rate-determining step.
(b) Show how the proposed mechanism leads to the given rate equation.
(c) State one reason why Z must not appear in the final rate equation.
(5 marks)
(a)
Correct explanation that Step 1 is the slowest step and therefore controls the overall rate (1 mark)
(b)
Recognition that the rate depends on reactants in the slow step only (1 mark)
Correct linkage between Step 1 and rate = k[X][Y] (1 mark)
(c)
Correct statement that Z is an intermediate and is not present at the start of the reaction (1 mark)
Correct statement that intermediates must not appear in the final rate equation (1 mark)

