OCR Specification focus:
‘Define rate, order, overall order, rate constant, half-life; deduce orders and write rate = k[A]^m[B]^n.’
Chemical kinetics examines how fast reactions occur, why rates vary, and how experimental data reveals relationships between reactant concentrations and reaction speed.
Rate of Reaction
The rate of reaction describes how quickly reactants are converted into products. It is a central concept in kinetics and underpins all rate equations and graphical analyses.
Rate of reaction: The change in concentration of a reactant or product per unit time, usually measured in mol dm⁻³ s⁻¹.
In practice, rates can be expressed as the decrease in concentration of a reactant or the increase in concentration of a product. The sign is usually ignored, as rate refers to speed rather than direction.
Rates are affected by several factors, including concentration, temperature, pressure (for gases), surface area, and catalysts. This subtopic focuses specifically on how concentration affects rate.
Rate Equations
A rate equation (also called a rate law) shows how the rate depends on the concentrations of reactants involved in the slow, rate-determining step of a reaction.
General Rate Equation = k[A]ᵐ[B]ⁿ
Rate = Rate of reaction (mol dm⁻³ s⁻¹)
k = Rate constant (units vary)
[A], [B] = Molar concentrations of reactants (mol dm⁻³)
m, n = Orders of reaction with respect to A and B
The overall structure of a rate equation must be deduced from experimental data and cannot usually be obtained directly from a balanced chemical equation.
Order of Reaction
The order of reaction indicates how the rate depends on the concentration of a particular reactant.
Order of reaction: The power to which the concentration of a reactant is raised in the rate equation.
Orders can be zero, first, second, or fractional, although OCR A-Level Chemistry focuses on zero, first, and second order reactions.
Zero Order
Rate is independent of reactant concentration
Changing concentration has no effect on rate
Common in reactions involving catalysts or saturated surfaces
In zero-order kinetics, the rate is constant and does not change as the reactant concentration falls.
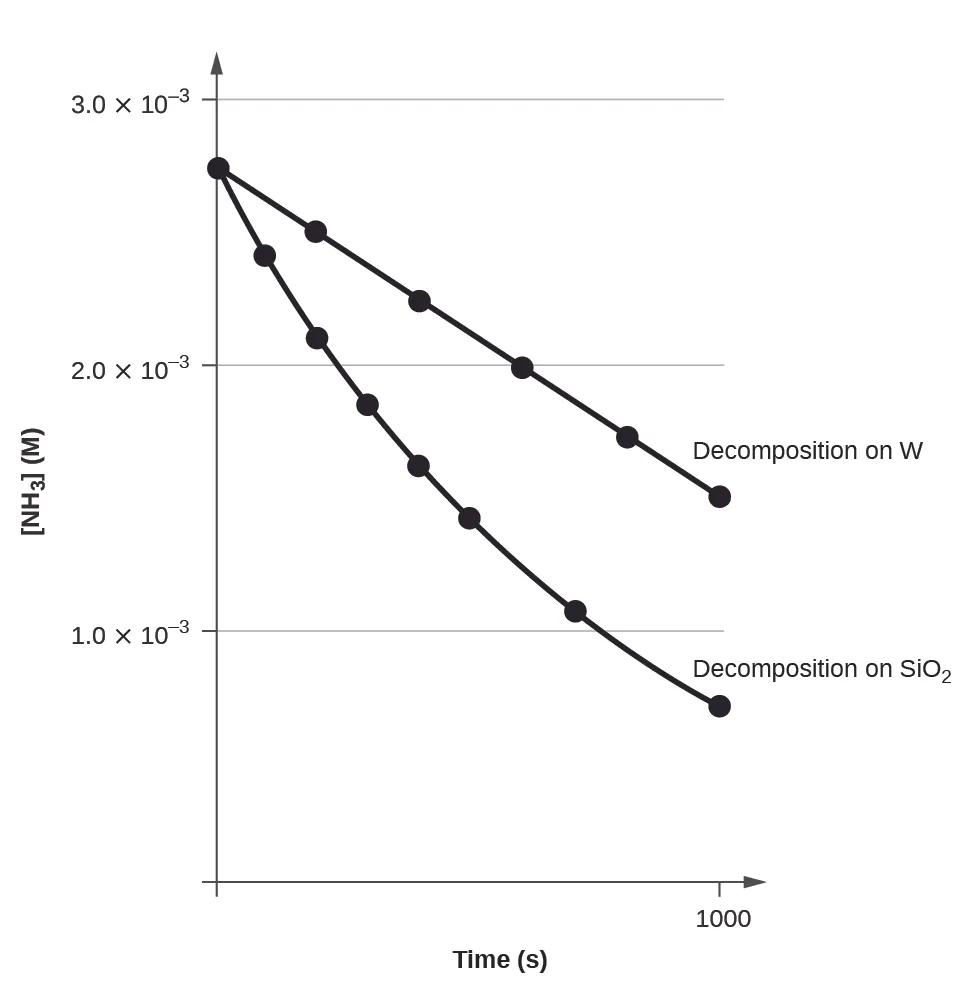
This graph compares zero-order and first-order concentration–time behaviour, showing constant-rate linear decrease versus exponential decay. The surface chemistry context is extra detail, but the order comparison directly supports understanding of reaction order. Source
Mathematically, the concentration term does not appear in the rate equation.
First Order
Rate is directly proportional to concentration
Doubling concentration doubles the rate
Many decomposition and radioactive decay processes follow first order kinetics
First order reactions are especially important because they exhibit a constant half-life.
Second Order
Rate depends on the square of one reactant concentration, or the product of two reactant concentrations
Doubling concentration increases rate by a factor of four
Overall Order
The overall order of reaction is found by adding together the individual orders of all reactants in the rate equation.
Overall order: The sum of the powers of the concentration terms in the rate equation.
For example, if rate = k[A]¹[B]², the overall order is three. The overall order gives insight into the complexity of the rate-determining step but does not necessarily match the stoichiometry of the equation.
Rate Constant, k
The rate constant links the rate of reaction to reactant concentrations and is specific to a particular reaction at a fixed temperature.
Rate constant (k): A proportionality constant in the rate equation that depends on temperature and the nature of the reaction.
The units of k vary depending on the overall order of the reaction. For example:
Zero order: mol dm⁻³ s⁻¹
First order: s⁻¹
Second order: dm³ mol⁻¹ s⁻¹
Although concentration affects rate, it does not change the value of k. Temperature changes, however, do alter k significantly.
Half-life
The half-life of a reaction is particularly important for first order processes.
Half-life: The time taken for the concentration of a reactant to decrease to half its initial value.

The beakers illustrate concentration halving at equal time intervals, a defining feature of first-order half-life behaviour. Colour is used purely as a visual aid and is not chemically significant. Source
For first order reactions, the half-life is constant and independent of the initial concentration. This property is used experimentally to identify first order kinetics. For zero and second order reactions, the half-life changes as the reaction proceeds.
Deducing Orders from Experimental Data
Orders of reaction must be deduced experimentally, commonly by comparing how rate changes when concentration is varied.
Reaction orders are found experimentally by measuring how the rate changes when reactant concentrations are changed.
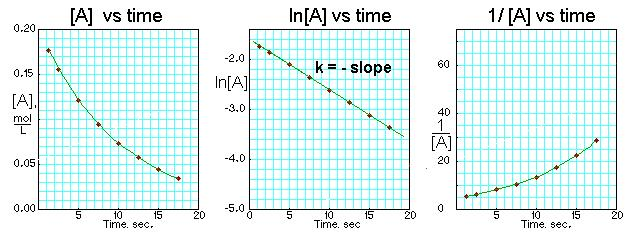
These plots show how first-order data produce a straight line when ln[A] is plotted against time. The use of integrated-rate-law graphs extends beyond this subsubtopic but reinforces what first-order behaviour looks like experimentally. Source
Key principles used include:
If doubling concentration doubles rate → first order
If doubling concentration quadruples rate → second order
If changing concentration has no effect → zero order
Only one reactant concentration is changed at a time, while others are kept constant, allowing the effect of each reactant to be isolated.
Writing Rate Equations
Once the orders are known, the rate equation can be written by:
Including only reactants that affect the rate
Raising each concentration term to its experimentally determined order
Combining all terms with the rate constant, k
The resulting equation summarises the kinetic behaviour of the reaction and forms the basis for further analysis in graphical and mechanistic studies later in the course.
FAQ
The stoichiometric equation shows overall reactant ratios, not how the reaction actually proceeds.
Reaction order depends on the rate-determining step, which may involve fewer particles than the full equation suggests.
As a result, the rate equation must be found experimentally and cannot usually be predicted from the balanced chemical equation alone.
Zero-order behaviour often indicates a limiting factor other than concentration.
Common reasons include:
A catalyst surface becoming saturated
All active sites being occupied
Light intensity limiting a photochemical reaction
In these cases, increasing reactant concentration has no effect on rate because the controlling factor is already operating at maximum capacity.
In first-order reactions, the rate is directly proportional to concentration.
As concentration decreases, the rate slows proportionally, meaning the time taken to halve the concentration remains the same.
This contrasts with zero- and second-order reactions, where the relationship between rate and concentration changes as the reaction proceeds.
Although fractional or negative orders exist in advanced kinetics, they are not required for OCR A-Level Chemistry.
At this level, students are expected to recognise:
Zero order
First order
Second order
These provide sufficient insight into how experimental data links concentration to rate without requiring complex mechanistic explanations.
The units of the rate constant depend on the overall order of the reaction.
This ensures that the rate equation always gives rate in mol dm⁻³ s⁻¹.
As the powers of concentration change, the units of k must adjust to maintain dimensional consistency, even though the physical meaning of k remains the same for a given reaction and temperature.
Practice Questions
Define the following terms as used in chemical kinetics:
a) Order of reaction
b) Rate constant
(2 marks)
a) Order of reaction (1 mark)
Definition stating it is the power to which the concentration of a reactant is raised in the rate equation.
b) Rate constant (1 mark)
Definition stating it is the proportionality constant in the rate equation at a given temperature.
A reaction between substances A and B is studied at constant temperature. The following experimental data are obtained.
Experiment 1:
[A] = 0.20 mol dm⁻³, [B] = 0.10 mol dm⁻³, rate = 4.0 × 10⁻³ mol dm⁻³ s⁻¹
Experiment 2:
[A] = 0.40 mol dm⁻³, [B] = 0.10 mol dm⁻³, rate = 8.0 × 10⁻³ mol dm⁻³ s⁻¹
Experiment 3:
[A] = 0.40 mol dm⁻³, [B] = 0.20 mol dm⁻³, rate = 8.0 × 10⁻³ mol dm⁻³ s⁻¹
a) Deduce the order of reaction with respect to A.
b) Deduce the order of reaction with respect to B.
c) Write the rate equation for the reaction.
d) State the overall order of the reaction.
(5 marks)
a) Order with respect to A (1 mark)
Correct identification that doubling [A] doubles the rate
First order with respect to A
b) Order with respect to B (1 mark)
Correct identification that doubling [B] has no effect on rate
Zero order with respect to B
c) Rate equation (2 marks)
Correct form: rate = k[A]
No inclusion of [B] term
d) Overall order (1 mark)
Correct statement that the overall order is 1

