OCR Specification focus:
‘Calculate amounts present at equilibrium; understand techniques to determine equilibrium quantities.’
Understanding equilibrium quantities is essential for analysing chemical systems, linking equilibrium constants to real reaction mixtures, and interpreting experimental data obtained from equilibrium investigations accurately.
Understanding Quantities at Equilibrium
At equilibrium, the forward and reverse reactions occur at equal rates, meaning the amounts of reactants and products remain constant over time. These constant amounts are referred to as equilibrium quantities and can be expressed in moles, concentrations, or partial pressures, depending on the system.
Equilibrium quantities are not necessarily equal amounts of reactants and products. Instead, they depend on:
The equilibrium constant for the reaction
The initial amounts of reactants
The stoichiometry of the balanced chemical equation
The conditions, particularly temperature
Understanding how to determine these quantities allows chemists to relate theoretical equilibrium expressions to measurable experimental data.
Describing Changes to Reach Equilibrium
When a reversible reaction is set up, it starts with known initial quantities. As the system moves towards equilibrium:
Reactant amounts typically decrease
Product amounts typically increase
The change in amount is governed by the stoichiometric ratios in the equation
Once equilibrium is reached, the remaining reactants and formed products constitute the equilibrium mixture. These values are used in equilibrium expressions such as Kc or Kp, although calculating these constants is addressed in other specification sections.
Measuring Equilibrium Quantities Experimentally
Equilibrium quantities cannot usually be measured directly and often require analytical techniques to determine concentrations or amounts of individual species in the equilibrium mixture.
Sampling the Equilibrium Mixture
To determine equilibrium quantities accurately:
The system must be allowed sufficient time to reach equilibrium
Conditions such as temperature must remain constant
Samples must be taken without disturbing the equilibrium position
For gaseous systems, equilibrium may be studied in sealed containers, while solutions are often sampled using volumetric techniques.
Titration as an Equilibrium Technique
One of the most common techniques for determining equilibrium quantities in solution is titration.
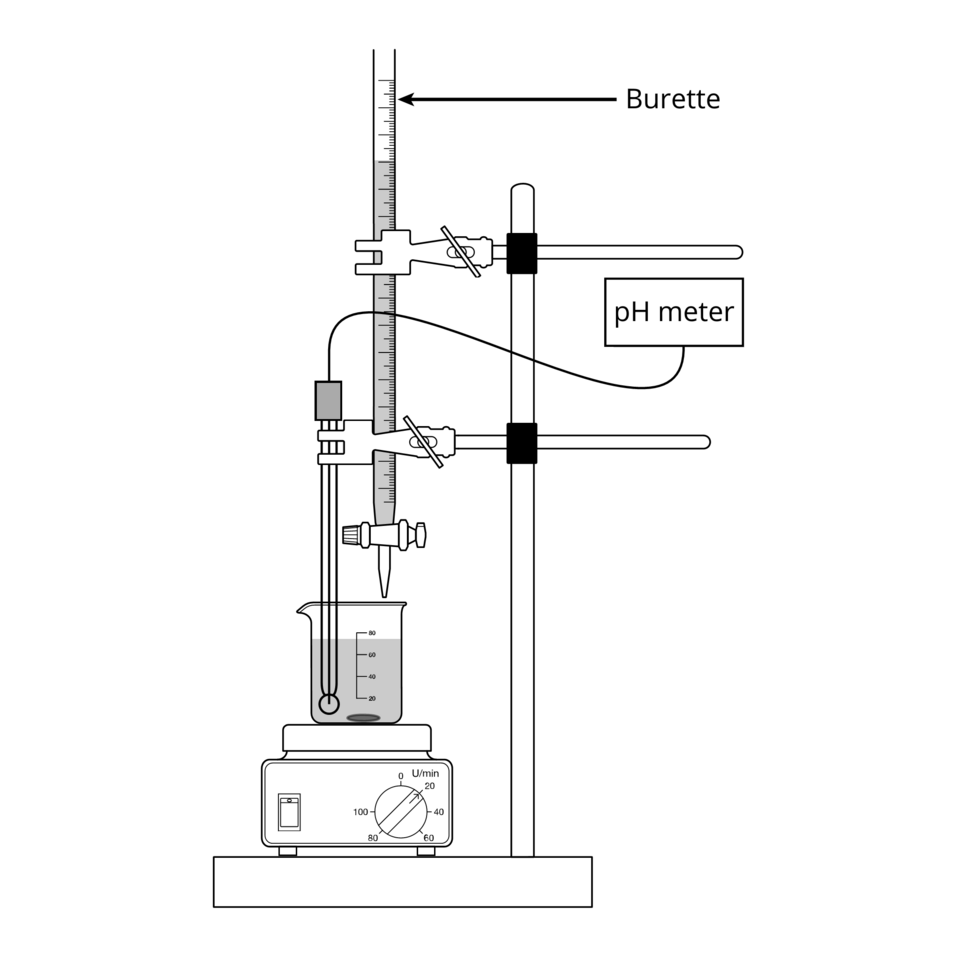
A labelled titration setup showing a burette delivering titrant into a flask containing the analyte, with mixing for consistent reaction. This is the standard arrangement used to determine the amount of a species in an equilibrium mixture by reacting it quantitatively. Source
This is particularly useful when one component of the equilibrium mixture reacts quantitatively with a known reagent.
Titration: A quantitative analytical technique in which a solution of known concentration is reacted with a solution of unknown concentration to determine its amount.
Titration is commonly used when:
One equilibrium species is an acid or base
The reacting species undergoes a complete reaction
A suitable indicator or pH meter can identify the end point
By measuring how much titrant reacts, the amount of a specific species at equilibrium can be deduced.
A normal explanatory sentence must follow this definition before another structured block is included. Titration remains a core laboratory method for equilibrium investigations due to its reliability and precision.
Colorimetry and Spectroscopy
Some equilibrium systems involve coloured species, making colorimetry a valuable technique.
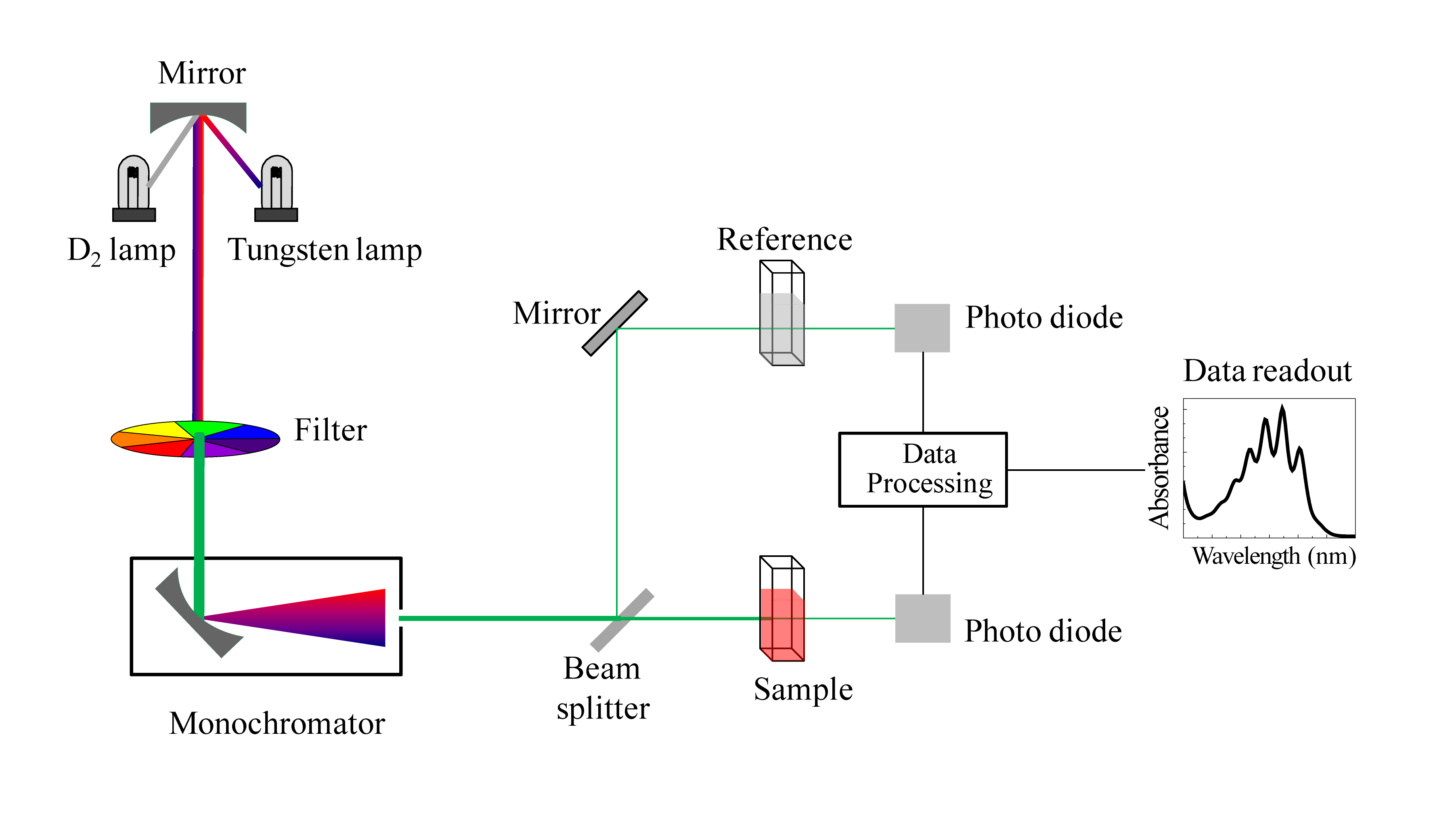
Schematic of a UV/visible spectrophotometer showing how light is wavelength-selected, passed through a sample cuvette and reference, and detected to measure absorbance. The dual-beam layout goes beyond basic colorimetry but clarifies how instruments compare sample and reference signals. Source
Colorimetry works by:
Passing light of a specific wavelength through a solution
Measuring the absorbance, which is proportional to concentration
Comparing results to a calibration curve
This method is especially useful when:
One equilibrium species is strongly coloured
The system does not interfere with light absorption
The equilibrium is rapid and stable during measurement
Colorimetry allows continuous or repeated measurements without significantly disturbing the equilibrium mixture.
Gas Phase Equilibria Techniques
For gaseous equilibria, equilibrium quantities are often determined using:
Pressure measurements
Gas syringe volume readings
Mass changes in closed systems

Diagram illustrating Dalton’s law of partial pressures, showing how individual gas pressures add to give total pressure. The atmospheric gas example adds extra context, but the partial-pressure concept is directly applicable to gas-phase equilibrium analysis. Source
By measuring total pressure and using mole ratios from the balanced equation, partial pressures and amounts of individual gases can be determined.
Partial pressure: The pressure exerted by a single gas in a mixture of gases, proportional to its mole fraction.
At least one normal sentence must follow this definition. Gas equilibrium techniques rely heavily on accurate pressure and volume data under controlled temperature conditions.
Using Equilibrium Data to Determine Quantities
Once experimental measurements are obtained, chemists can:
Convert measured values into moles or concentrations
Use stoichiometry to relate different species
Identify the equilibrium composition of the system
Although full calculations are addressed elsewhere, students must understand that equilibrium quantities always reflect a balance between reactants and products, rather than complete reaction.
Sources of Experimental Uncertainty
When determining equilibrium quantities, accuracy may be affected by:
Incomplete equilibration
Temperature fluctuations
Loss of gases or solution during sampling
Indicator or instrumental limitations
Understanding these limitations helps in evaluating the reliability of equilibrium data and choosing the most appropriate experimental technique.
Importance of Techniques in Equilibrium Chemistry
The ability to determine equilibrium quantities underpins much of physical chemistry. These techniques allow chemists to:
Test theoretical predictions
Compare different equilibrium systems
Investigate the effects of changing conditions
Mastery of equilibrium techniques ensures that equilibrium constants and positions are grounded in measurable chemical reality, supporting deeper understanding across the topic.
FAQ
If measurements are taken before equilibrium is established, the amounts of reactants and products will still be changing. This leads to incorrect values that do not represent the true equilibrium state.
Allowing sufficient time ensures that the forward and reverse reactions have equal rates, so the measured quantities are stable and meaningful for equilibrium analysis.
A selective analytical method is required that responds to only one species. This may involve:
Choosing a reagent that reacts with only one component
Using a wavelength in colorimetry where only one species absorbs light
Applying prior separation steps before analysis
These approaches allow one equilibrium quantity to be measured without interference from others.
Temperature affects both the position of equilibrium and the rate at which equilibrium is reached. Even small temperature changes can alter the amounts of reactants and products present.
Maintaining constant temperature ensures that the equilibrium composition remains unchanged during measurement, improving the reliability of the results.
Instrumental methods such as colorimetry can provide rapid, repeatable, and precise measurements. They also allow multiple readings to be taken without significantly disturbing the equilibrium mixture.
This makes them particularly useful for equilibria involving low concentrations or small changes in composition.
Many equilibrium species cannot be isolated or measured on their own. Instead, their quantities are deduced from measurable properties such as volume, pressure, absorbance, or reaction with a known reagent.
Indirect measurement allows equilibrium systems to be studied without breaking or shifting the equilibrium itself.
Practice Questions
A reversible reaction reaches equilibrium in solution. State what is meant by equilibrium quantities and explain why they remain constant once equilibrium is established.
(2 marks)
1 mark for stating that equilibrium quantities are the amounts or concentrations of reactants and products present at equilibrium
1 mark for explaining that they remain constant because the forward and reverse reactions occur at equal rates
Maximum 2 marks
A student investigates a reversible reaction that reaches equilibrium in aqueous solution.
(a) Describe one experimental technique that could be used to determine the quantity of a reactant present at equilibrium.
(b) Explain why the equilibrium position must not be disturbed when measuring equilibrium quantities.
(c) State one source of experimental uncertainty that could affect the accuracy of the measured equilibrium quantity.
(5 marks)
(a) Experimental technique (2 marks)
1 mark for naming a suitable technique such as titration or colorimetry
1 mark for describing how the technique determines the amount or concentration of a species at equilibrium
(b) Equilibrium disturbance (2 marks)
1 mark for stating that changing conditions or removing substances would shift the equilibrium position
1 mark for explaining that this would change the equilibrium quantities being measured
(c) Experimental uncertainty (1 mark)
1 mark for stating a valid source of uncertainty, such as temperature changes, loss of sample, or indicator error
Maximum 5 marks

