OCR Specification focus:
‘Write Kc and Kp for homogeneous and heterogeneous equilibria; omit constant solid and liquid terms.’
These notes explain how to construct equilibrium constant expressions for reactions, distinguishing Kc and Kp, handling phases correctly, and applying them consistently to chemical equilibria.
Equilibrium constants and chemical equilibria
An equilibrium constant is a numerical value that describes the position of equilibrium for a reversible reaction at a fixed temperature. It links the equilibrium composition of a system to its balanced chemical equation. For OCR A-Level Chemistry, the focus is on constructing correct expressions rather than performing calculations.
The value of an equilibrium constant depends only on temperature and the stoichiometry of the equation written. Changing concentrations, pressure, or adding a catalyst does not change its value, provided temperature is constant.
The equilibrium constant Kc
Kc is used when equilibrium concentrations are expressed in mol dm⁻³. It applies to reactions in solution or gases where concentration data are used.
Kc: The equilibrium constant expressed using equilibrium concentrations of reactants and products, each raised to the power of their stoichiometric coefficients.
A Kc expression is derived directly from the balanced chemical equation. For a general reaction:
aA + bB ⇌ cC + dD
the equilibrium constant expression follows a strict pattern.
Equilibrium constant (Kc) = [C]ᶜ[D]ᵈ / [A]ᵃ[B]ᵇ
[C], [D], [A], [B] = equilibrium concentrations in mol dm⁻³
a, b, c, d = stoichiometric coefficients
For a general equilibrium aA + bB ⇌ cC + dD, Kc is written as products over reactants, with each term raised to its stoichiometric coefficient.
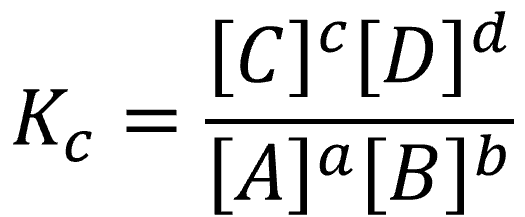
This diagram shows the general form of an equilibrium constant in concentration terms, Kc, for a reaction aA + bB ⇌ cC + dD. The powers match the balancing coefficients, and only species with variable concentration belong in the expression. Source
Only species whose concentrations change affect the equilibrium position and are included in the expression. Concentrations must be equilibrium values, not initial values.
Homogeneous equilibria and Kc
A homogeneous equilibrium is one in which all reactants and products are in the same physical state, typically all gases or all aqueous species.
In homogeneous equilibria:
All gaseous or aqueous species are included in the Kc expression.
Each concentration term is raised to the power shown in the balanced equation.
The physical state symbols do not appear in the Kc expression but determine inclusion.
For example, equilibria involving gases or ions in solution are commonly treated using Kc, provided concentration data are available.
Heterogeneous equilibria and omission of solids and liquids
A heterogeneous equilibrium involves species in different physical states. In these cases, not all substances appear in the equilibrium constant expression.
Pure solids and pure liquids are omitted from Kc expressions because:
Their concentrations remain constant during the reaction.
They are incorporated into the value of the equilibrium constant.
Including them would not reflect changes in equilibrium position.
This rule applies regardless of the stoichiometric coefficients of solids or liquids in the equation. Only gases and aqueous species are included in Kc expressions.
In heterogeneous equilibria, pure solids and pure liquids are omitted from Kc and Kp expressions because their concentrations are effectively constant.
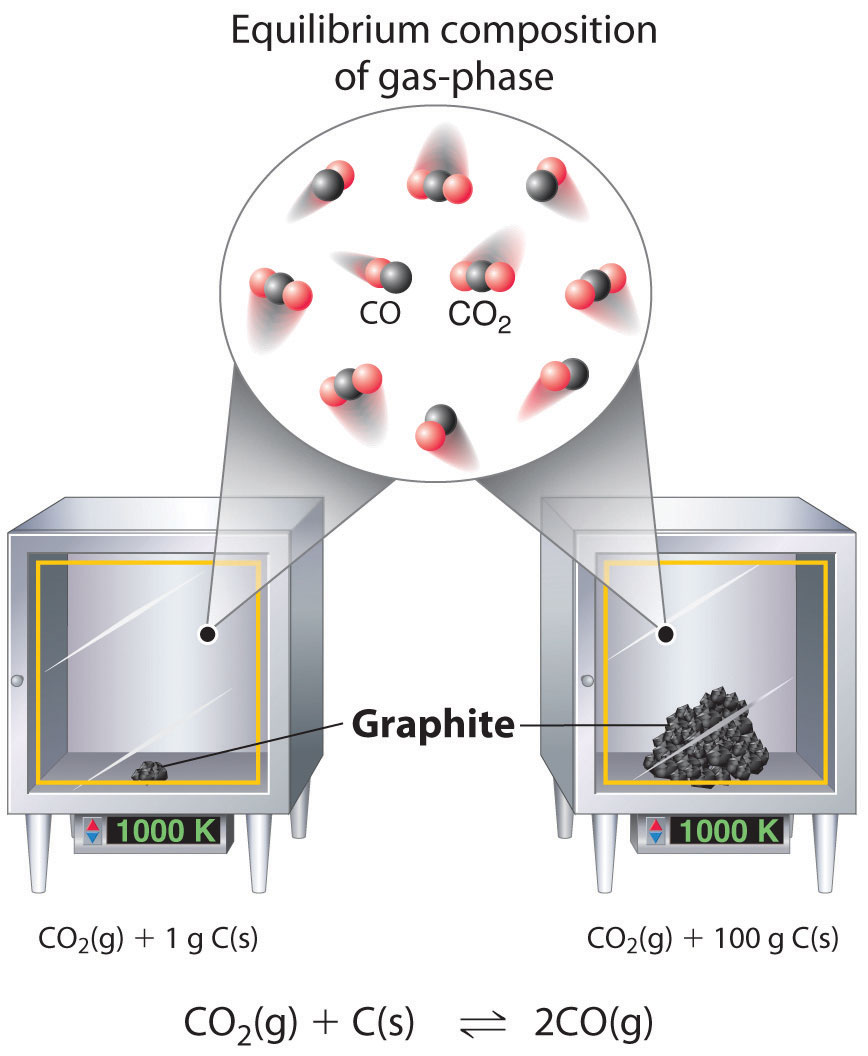
This figure illustrates a heterogeneous equilibrium where a solid is present in different amounts but the equilibrium gas composition is unchanged at the same temperature. It supports why solids are not included in K expressions: their activity is treated as constant. Source
The equilibrium constant Kp
Kp is used for equilibria involving gases where equilibrium partial pressures are measured, rather than concentrations.
Kp: The equilibrium constant expressed using equilibrium partial pressures of gaseous reactants and products, each raised to the power of their stoichiometric coefficients.
Kp is particularly useful for gas-phase equilibria studied using pressure data.
Equilibrium constant (Kp) = (pC)ᶜ(pD)ᵈ / (pA)ᵃ(pB)ᵇ
p = equilibrium partial pressure in kPa
a, b, c, d = stoichiometric coefficients
As with Kc, the expression is written using the balanced chemical equation, and only equilibrium values are used.
Inclusion rules for Kp expressions
When writing Kp expressions:
Only gaseous species are included.
Solids and liquids are omitted for the same reasons as in Kc expressions.
Each partial pressure is raised to the appropriate power from the equation.
Kp cannot be used for equilibria involving only solids and liquids, as no gaseous species are present to contribute partial pressures.
Comparing Kc and Kp
Kc and Kp describe the same equilibrium position but use different measurable quantities. The choice between them depends on how equilibrium data are obtained.
Key distinctions include:
Kc uses concentrations (mol dm⁻³).
Kp uses partial pressures (kPa).
Both depend only on temperature.
Both omit solids and pure liquids from expressions.
Although Kc and Kp are related mathematically, students are expected to focus on constructing correct expressions rather than converting between them at this stage.
Stoichiometry and powers in equilibrium expressions
The powers applied to concentration or pressure terms come directly from the coefficients in the balanced chemical equation. Correct balancing is therefore essential before writing Kc or Kp.
Important points to remember:
Changing the equation changes the equilibrium constant expression.
Reversing the equation inverts the equilibrium constant.
Multiplying the equation by a factor raises the equilibrium constant to that power.
These relationships reinforce the importance of linking equilibrium constants to the exact chemical equation used.
Common errors to avoid
Students frequently make avoidable mistakes when writing equilibrium expressions. Typical errors include:
Including solids or liquids in Kc or Kp expressions.
Using initial instead of equilibrium values.
Omitting powers or using incorrect stoichiometric coefficients.
Mixing concentration and pressure terms in the same expression.
Careful attention to physical states and correct use of equilibrium quantities ensures expressions remain fully consistent with the OCR specification.
FAQ
In a heterogeneous equilibrium, the concentration of a pure solid does not change as the reaction proceeds.
Because its concentration is effectively constant, it is incorporated into the value of the equilibrium constant rather than written explicitly.
This is why adding more solid may shift the position of equilibrium temporarily but does not alter the numerical value of Kc or Kp at a fixed temperature.
Yes, gas-phase equilibria can be described using either Kc or Kp.
The choice depends on whether equilibrium data are given as concentrations or partial pressures.
Both constants describe the same equilibrium position at the same temperature, but they are expressed using different measurable quantities.
The powers in Kc and Kp expressions come directly from the stoichiometric coefficients.
If the equation is not correctly balanced, the powers in the equilibrium expression will be incorrect.
This leads to an incorrect equilibrium constant that does not reflect the true equilibrium composition of the system.
Reversing a chemical equation inverts the equilibrium constant.
This means the new equilibrium constant is equal to 1 divided by the original value.
This occurs because the positions of products and reactants in the expression are swapped.
Kp is defined using partial pressures, which are only meaningful for gases.
Liquids have fixed density and do not exert partial pressure in the same way.
As a result, liquids are omitted from Kp expressions for the same reason as solids: their effect is already built into the value of the constant.
Practice Questions
The reaction below establishes an equilibrium in the gas phase:
2SO2(g) + O2(g) ⇌ 2SO3(g)
Write the expression for the equilibrium constant Kp for this reaction.
(2 marks)
Correct identification of products over reactants in the Kp expression: 1 mark
Correct use of stoichiometric powers, including squared terms for SO2 and SO3: 1 mark
Acceptable answer:
Kp = (pSO3)² / (pSO2)²(pO2)
Calcium carbonate decomposes on heating according to the equation:
CaCO3(s) ⇌ CaO(s) + CO2(g)
a) State which substances are omitted from the equilibrium constant expression and explain why.
b) Write the expression for the equilibrium constant Kp for this reaction.
(5 marks)
a) Omitted substances and explanation (3 marks)
Correctly states that CaCO3(s) and CaO(s) are omitted: 1 mark
States that they are solids: 1 mark
Explains that solids have constant concentration (or constant activity) and therefore do not affect the value of the equilibrium constant: 1 mark
b) Kp expression (2 marks)
Correct identification that only CO2(g) appears in the Kp expression: 1 mark
Correct expression written with no denominator terms: 1 mark
Acceptable answer:
Kp = pCO2

