OCR Specification focus:
‘Explain temperature effects on equilibrium constants and their constancy with concentration, pressure or catalysts.’
This topic explains how equilibrium constants respond to temperature changes while remaining unaffected by concentration, pressure, or catalysts, clarifying common misconceptions about equilibrium behaviour.
Understanding Equilibrium Constants
An equilibrium constant provides a quantitative measure of the position of equilibrium for a reversible reaction at a fixed temperature. For OCR A-Level Chemistry, it is essential to understand what factors do and do not affect its value.
The equilibrium constant is derived from the equilibrium law and applies once dynamic equilibrium has been established. Its magnitude indicates whether reactants or products are favoured at equilibrium, but it does not show how quickly equilibrium is reached.
Temperature and Equilibrium Constants
Temperature is the only factor that changes the numerical value of an equilibrium constant. This occurs because temperature alters the relative energies of reactants and products.
Changing temperature changes the value of the equilibrium constant, unlike changes in concentration, pressure, or catalysts.
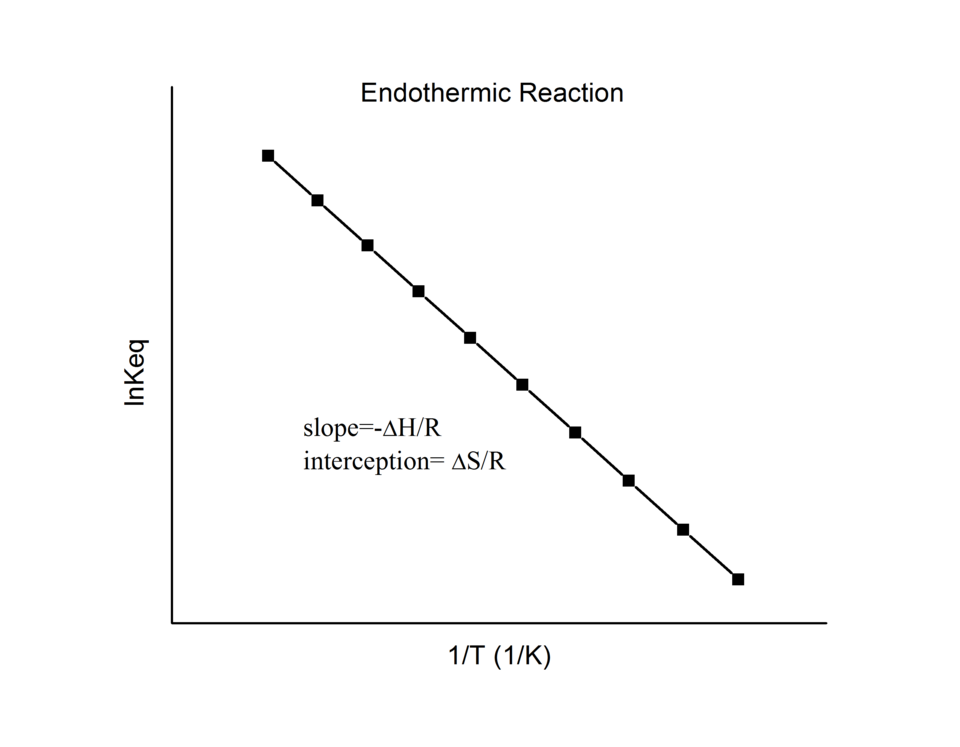
This van ’t Hoff plot illustrates how the equilibrium constant varies with temperature for an endothermic reaction. As temperature increases, the equilibrium constant increases, indicating greater product formation at equilibrium. Source
Effect of Temperature on Endothermic Equilibria
For an endothermic forward reaction, heat is absorbed as the reaction proceeds.
Increasing temperature supplies additional energy.
The equilibrium position shifts to favour the endothermic direction.
The equilibrium constant increases, indicating a greater proportion of products at equilibrium.
Lowering the temperature has the opposite effect, favouring the exothermic reverse reaction and reducing the value of the equilibrium constant.
Effect of Temperature on Exothermic Equilibria
For an exothermic forward reaction, heat is released.
Increasing temperature adds heat to the system.
The equilibrium position shifts to favour the endothermic reverse reaction.
The equilibrium constant decreases, showing fewer products relative to reactants.
Cooling the system favours the exothermic forward reaction, increasing the equilibrium constant.
Link Between Enthalpy Change and K
The effect of temperature on the equilibrium constant depends on the enthalpy change (ΔH) of the reaction.
Enthalpy change (ΔH): The energy absorbed or released when reactants are converted to products at constant pressure.
If ΔH is positive (endothermic), increasing temperature increases K.
If ΔH is negative (exothermic), increasing temperature decreases K.
This relationship explains why equilibrium constants are always quoted at a specific temperature, commonly 298 K.
Why Concentration Does Not Affect K
Changing the concentration of reactants or products can shift the position of equilibrium but does not change the value of the equilibrium constant.
Increasing reactant concentration causes the equilibrium to shift right.
Increasing product concentration causes the equilibrium to shift left.
In both cases, the system adjusts until the ratio defined by K is restored.
The equilibrium constant depends only on temperature, not on how equilibrium is disturbed.
Pressure and Equilibrium Constants
For gaseous equilibria, changing pressure can affect equilibrium position but not the equilibrium constant.
Increasing pressure favours the side with fewer moles of gas.
Decreasing pressure favours the side with more moles of gas.
These shifts alter equilibrium concentrations or partial pressures, but once equilibrium is re-established at the same temperature, the value of K remains unchanged.
This distinction is critical: equilibrium position may change, but K does not.
Catalysts and Equilibrium Constants
A catalyst speeds up both the forward and reverse reactions equally.
Catalyst: A substance that increases reaction rate without being consumed, by providing an alternative pathway with lower activation energy.
A catalyst does not change the value of K; it only helps the system reach equilibrium faster.
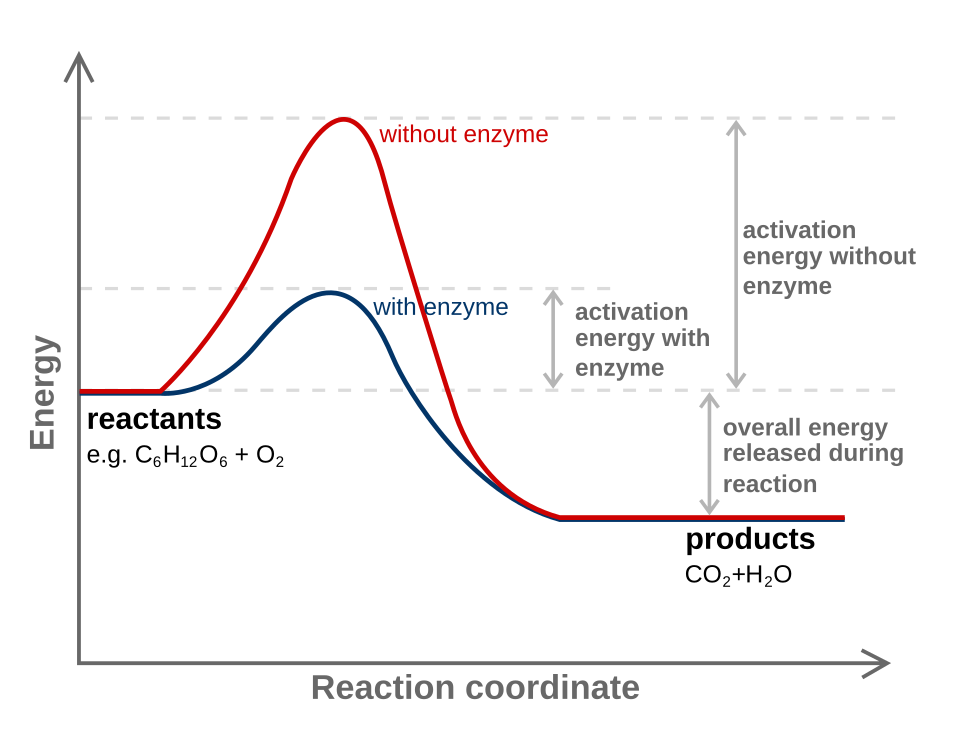
The diagram shows that a catalyst lowers activation energy but does not change the energy difference between reactants and products. As a result, the equilibrium constant remains unchanged while equilibrium is reached more quickly. Source
A catalyst allows equilibrium to be reached more quickly.
It does not alter the energies of reactants or products.
The equilibrium constant remains unchanged.
Catalysts affect rate, not equilibrium position or K.
Common Misconceptions Addressed
It is important to separate the ideas of equilibrium position and equilibrium constant.
Changing concentration, pressure, or adding a catalyst does not change K.
These factors only influence how the system moves to restore equilibrium.
Only temperature affects the relative stability of reactants and products, and therefore K.
Students often confuse shifts in equilibrium with changes in K; OCR assessments regularly test this distinction.
Significance of Constant K Values
At a given temperature, the equilibrium constant provides a reliable reference point.
A large K indicates products are favoured.
A small K indicates reactants are favoured.
A K value close to 1 suggests significant amounts of both reactants and products.
Understanding what does and does not affect K ensures correct interpretation of equilibrium data and avoids incorrect assumptions when conditions change.
Changing concentration (or pressure) shifts the position of equilibrium, but at the same temperature the system returns to a state where Q = K and K is unchanged.
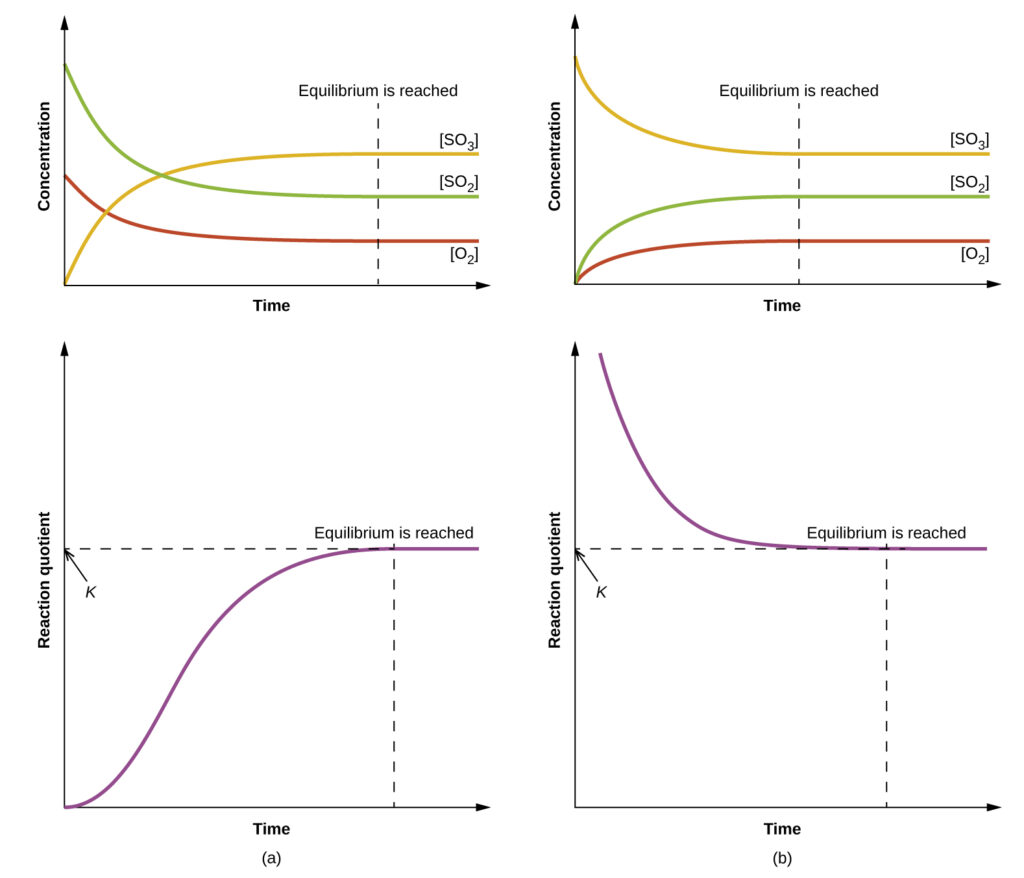
The figure shows that systems starting with different initial concentrations still reach equilibrium where Q equals K at the same temperature. This reinforces that equilibrium constants are independent of initial concentrations. Source
Summary of Key Effects on Equilibrium Constants
Temperature: changes the value of K.
Concentration: no effect on K.
Pressure: no effect on K.
Catalysts: no effect on K.
This principle underpins equilibrium control in chemical systems and is central to OCR A-Level Chemistry equilibrium questions.
FAQ
The equilibrium constant depends on the relative energies of reactants and products, which change with temperature.
Because this energy balance is temperature-dependent, a value of K is only valid at the temperature at which it was measured.
If the temperature changes, a new equilibrium constant must be determined, even if all other conditions remain the same.
The size of K indicates how far a reaction lies towards products or reactants at equilibrium.
Very large K values mean products are strongly favoured.
Very small K values indicate reactants dominate.
However, even extreme values do not imply a reaction is complete; both reactants and products are always present at equilibrium.
Changing pressure alters the partial pressures of gases, causing the equilibrium position to shift.
Once equilibrium is re-established at the same temperature, the ratio of equilibrium partial pressures returns to the same value.
As Kp is defined using these ratios, its value remains constant despite pressure changes.
The sign of ΔH indicates whether heat is absorbed or released in the forward reaction.
Endothermic reactions have positive ΔH and higher temperatures increase K.
Exothermic reactions have negative ΔH and higher temperatures decrease K.
This allows predictions about K without calculating its numerical value.
Yes, equilibrium constants measured at the same temperature can be compared directly.
A reaction with a larger K is more product-favoured at equilibrium than one with a smaller K.
However, comparisons are only valid if the temperature is identical, as temperature changes affect K differently for each reaction.
Practice Questions
For a reversible reaction at equilibrium, state which of the following changes will alter the value of the equilibrium constant, K, and which will not. Explain your answer briefly.
Increasing the temperature
Adding a catalyst
(2 marks)
1 mark: Correctly states that increasing temperature changes the value of K.
1 mark: Correctly states that adding a catalyst does not change the value of K, with explanation that it affects rate only or speeds up both forward and reverse reactions equally.
A reversible reaction is carried out in a closed system at a fixed temperature.
(a) Explain why changing the concentration of a reactant causes the position of equilibrium to change but does not change the value of the equilibrium constant, K.
(b) The forward reaction is exothermic. Explain the effect of increasing temperature on:
(i) the position of equilibrium
(ii) the value of the equilibrium constant, K
(5 marks)
(a) (2 marks)
1 mark: States that changing concentration disturbs the equilibrium and causes a shift to oppose the change (Le Chatelier’s principle).
1 mark: States that once equilibrium is re-established at the same temperature, the ratio of equilibrium concentrations returns to the same value, so K is unchanged.
(b) (3 marks)
(i) (1 mark)
1 mark: Correctly states that increasing temperature shifts the equilibrium in the endothermic direction, favouring the reverse reaction.
(ii) (2 marks)
1 mark: States that the value of K decreases when temperature is increased for an exothermic reaction.
1 mark: Explains that this is because temperature affects the relative energies of reactants and products, altering the equilibrium constant.
Maximum of 5 marks available for Question 2.

