OCR Specification focus:
‘Use mole fraction and partial pressure terminology for gaseous equilibria.’
Understanding gaseous equilibria requires describing gas mixtures quantitatively, using mole fractions and partial pressures to relate composition to equilibrium expressions and chemical behaviour accurately.
Gaseous Mixtures in Chemical Equilibria
In many equilibria studied at A-Level, reactants and products are gases contained in the same system. Unlike solids and liquids, gases mix completely and occupy the entire volume of their container. As a result, the behaviour of each gas depends not only on its own amount, but also on the total amount of gas present.
To describe such systems precisely, chemists use mole fraction and partial pressure. These concepts allow the contribution of each gas to be quantified independently, which is essential for understanding equilibrium constants such as Kp.
Mole Fraction
The mole fraction expresses how much of a mixture is made up by a particular gas, relative to the total number of moles present.
Mole fraction (χ): The ratio of the number of moles of a given gas to the total number of moles of all gases in the mixture.
Mole fraction has no units because it is a ratio. For a mixture of gases A, B, and C:
The mole fraction of A depends only on the number of moles of A and the total moles present.
The sum of the mole fractions of all gases in a mixture is always equal to 1.
This reflects the idea that the entire gas mixture is fully accounted for by its individual components.
Mole fraction is particularly useful because it is independent of temperature and pressure, provided the gases behave ideally. This makes it a convenient way to describe composition in changing conditions.
Partial Pressure
Each gas in a mixture behaves as if it alone occupies the container, exerting its own pressure. This individual contribution is known as the partial pressure.
Partial pressure: The pressure that a single gas would exert if it occupied the container alone at the same temperature and volume.
Partial pressure is measured in pascals (Pa) or kilopascals (kPa), consistent with standard pressure units used in equilibrium calculations.
In a gas mixture:
The total pressure is the sum of the partial pressures of all gases.
Each gas contributes to the total pressure in proportion to its mole fraction.
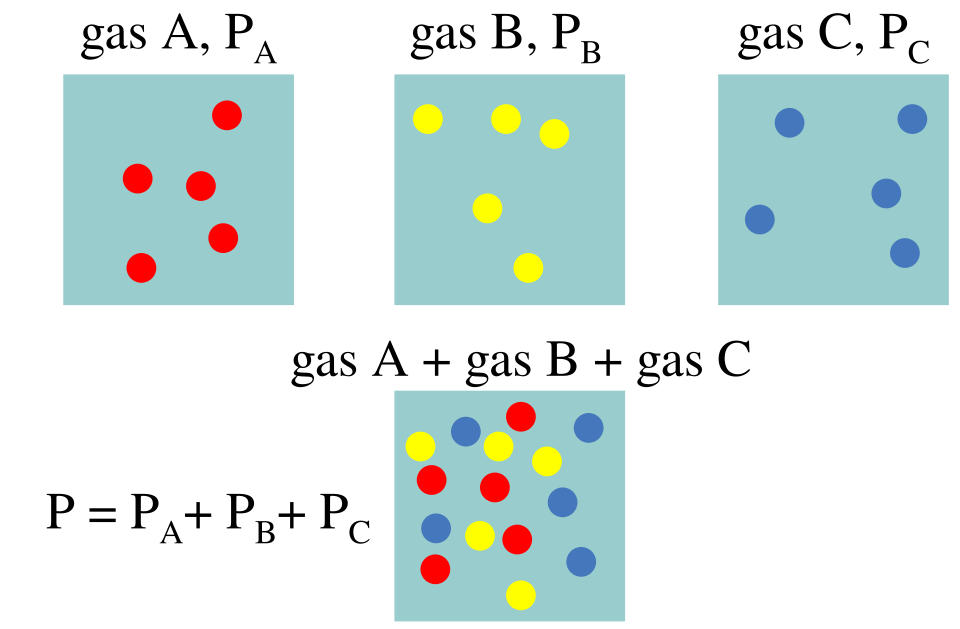
This schematic shows gases A, B, and C in separate containers, each exerting its own partial pressure. When combined at the same conditions, the total pressure is the sum of the individual partial pressures, illustrating Dalton’s law. Source
Relationship Between Mole Fraction and Partial Pressure
Mole fraction and partial pressure are directly linked through the total pressure of the system.
Partial pressure of a gas (p) = χ × Pₜₒₜₐₗ
χ = Mole fraction of the gas (no units)
Pₜₒₜₐₗ = Total pressure of the gas mixture (Pa or kPa)
This relationship shows that:
A gas with a larger mole fraction exerts a larger partial pressure.
If the total pressure changes, the partial pressures change proportionally, provided mole fractions remain constant.
This equation is fundamental when working with gaseous equilibria, as equilibrium constants depend on partial pressures rather than mole fractions alone.
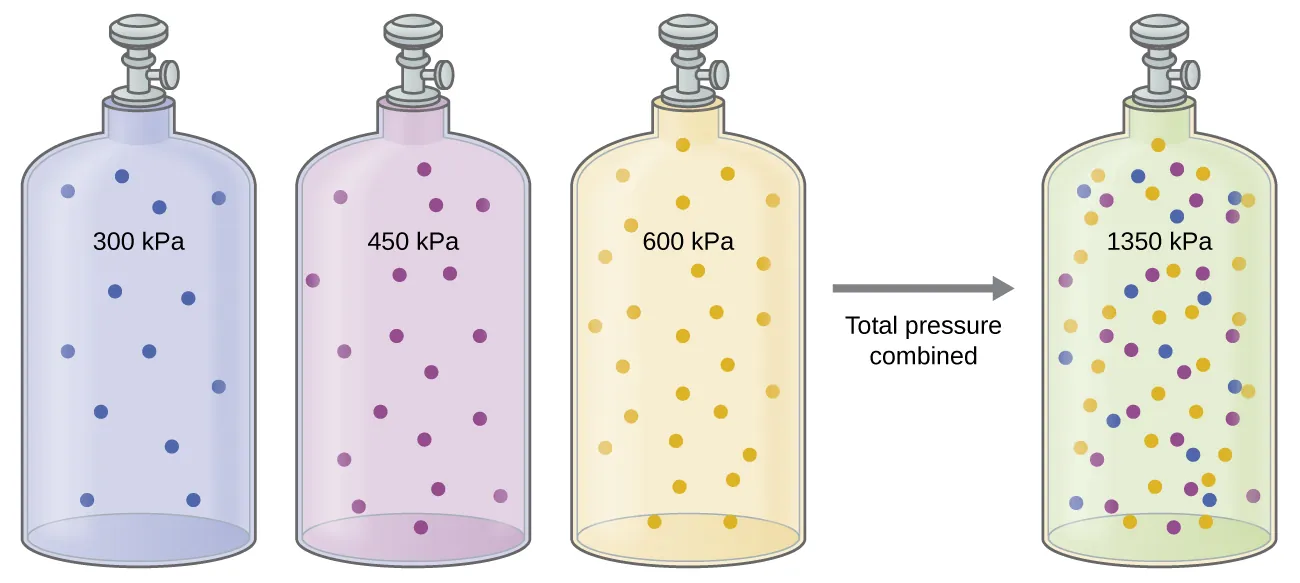
Three equal-volume cylinders containing gases at different pressures are combined into one cylinder. The total pressure equals the sum of the individual pressures, demonstrating how partial pressures contribute to total pressure in a gas mixture. Source
Partial Pressure and Gaseous Equilibria
In equilibria involving gases, the position of equilibrium depends on the partial pressures of reactants and products, not their individual amounts or concentrations.
For example:
Increasing the partial pressure of a gaseous reactant tends to favour the forward reaction.
Decreasing the partial pressure of a product can shift equilibrium towards further product formation.
These effects are explained by Le Chatelier’s principle, but the quantitative description relies on partial pressures.
Importance for Kp Expressions
The equilibrium constant Kp is defined using partial pressures of gaseous species at equilibrium.
Each gas appears in the Kp expression raised to the power of its stoichiometric coefficient.
Partial pressures are used instead of concentrations because gases are compressible and their behaviour depends strongly on pressure.
Understanding how partial pressures are obtained from mole fractions and total pressure is therefore essential before attempting Kp calculations.
Ideal Gas Assumption
At A-Level, gases are assumed to behave as ideal gases unless stated otherwise. Under this assumption:
Gas particles have negligible volume.
There are no intermolecular forces between particles.
The relationship between mole fraction and partial pressure holds exactly.
This allows mole fraction and partial pressure concepts to be applied consistently across different equilibrium systems without correction factors.
Practical Context and Terminology
When describing gaseous equilibria, correct terminology is essential:
Always specify partial pressure, not simply “pressure”, when referring to an individual gas.
Use mole fraction to describe composition rather than percentage, as it links directly to equilibrium expressions.
Remember that solids and liquids do not have partial pressures and are excluded from gas-phase equilibrium descriptions.
Accurate use of mole fraction and partial pressure terminology underpins all further work on equilibrium constants and equilibrium position in gaseous systems.
FAQ
Mole fraction is directly linked to the number of particles present, which is what determines gas behaviour at equilibrium.
Because partial pressure is proportional to mole fraction, using mole fraction allows equilibrium expressions to be written cleanly without extra conversion steps.
Percentages can be converted into mole fractions, but mole fraction avoids unnecessary arithmetic and reduces the risk of errors in equilibrium work.
If the mole fractions remain constant, each gas’s partial pressure changes in direct proportion to the total pressure.
Increasing total pressure increases all partial pressures
Decreasing total pressure decreases all partial pressures
This is important when considering compression or expansion of a gas mixture at equilibrium.
No. At the same temperature, all gases exert pressure based on the number of moles present, not their molar mass.
A heavier gas and a lighter gas with the same number of moles will exert equal partial pressures under identical conditions.
This behaviour follows the ideal gas model used at A-Level.
Gases are compressible, so their concentrations change significantly with pressure.
Partial pressure accounts directly for both the amount of gas and the volume it occupies, making it a more reliable variable for describing gas equilibria.
This is why Kp is defined using partial pressures rather than concentrations.
Yes. Partial pressure describes the physical state of a gas mixture, regardless of whether a chemical equilibrium has been established.
However, partial pressures are only substituted into equilibrium expressions once the system has reached equilibrium.
Before equilibrium, partial pressures can still be measured and used to predict how the system will change.
Practice Questions
A gas mixture contains nitrogen and hydrogen only. The mole fraction of nitrogen is 0.75.
(a) State the mole fraction of hydrogen.
(b) State the unit of mole fraction.
(2 marks)
(a) Mole fraction of hydrogen = 0.25
1 mark for correct value
(b) No units
1 mark for stating that mole fraction is unitless
A sealed container holds a mixture of three gases, A, B and C, at constant temperature.
The total pressure of the mixture is 200 kPa.
The mole fractions of gases A and B are 0.40 and 0.35 respectively.
(a) Calculate the mole fraction of gas C.
(b) Explain what is meant by the term partial pressure.
(c) State the partial pressure of gas A and explain how it is obtained from the mole fraction.
(5 marks)
(a) Mole fraction of gas C = 0.25
1 mark for subtracting mole fractions of A and B from 1
Accept 1 − (0.40 + 0.35)
(b) Partial pressure definition
1 mark for stating that it is the pressure a gas would exert if it occupied the container alone
1 mark for reference to same temperature and volume
(c) Partial pressure of gas A = 80 kPa
1 mark for correct calculation using mole fraction × total pressure
1 mark for explanation that partial pressure is proportional to mole fraction and total pressure

