OCR Specification focus:
'Calculate Kc and Kp, related quantities and their units without using quadratic equations.'
Equilibrium constants quantify the position of reversible reactions. Accurate calculation of Kc and Kp, including correct units, is essential for understanding chemical equilibria.
Understanding Equilibrium Constant Calculations
Equilibrium constants are numerical values that describe the ratio of products to reactants at equilibrium for a reversible reaction. In this subsubtopic, the focus is on calculating values of Kc and Kp, identifying their units, and manipulating equilibrium expressions without resorting to quadratic equations.
Kc and Kp are calculated using equilibrium concentrations or equilibrium partial pressures respectively. The structure of the equilibrium expression is fixed for a given reaction at a fixed temperature.
Calculating Kc from Equilibrium Data
Kc is used for reactions where equilibrium quantities are expressed in concentration (mol dm⁻³). To calculate Kc, equilibrium concentrations must be known or deduced.
Kc expression and calculation principles
The general approach involves:
Writing a balanced chemical equation.
Constructing the Kc expression using equilibrium concentrations.
Substituting equilibrium values into the expression.
Evaluating the numerical value of Kc.
Equilibrium constant (Kc): The ratio of the equilibrium concentrations of products to reactants, each raised to the power of their stoichiometric coefficients.
Always use equilibrium concentrations, not initial amounts or changes. Concentrations are raised to powers based on the balanced equation coefficients.
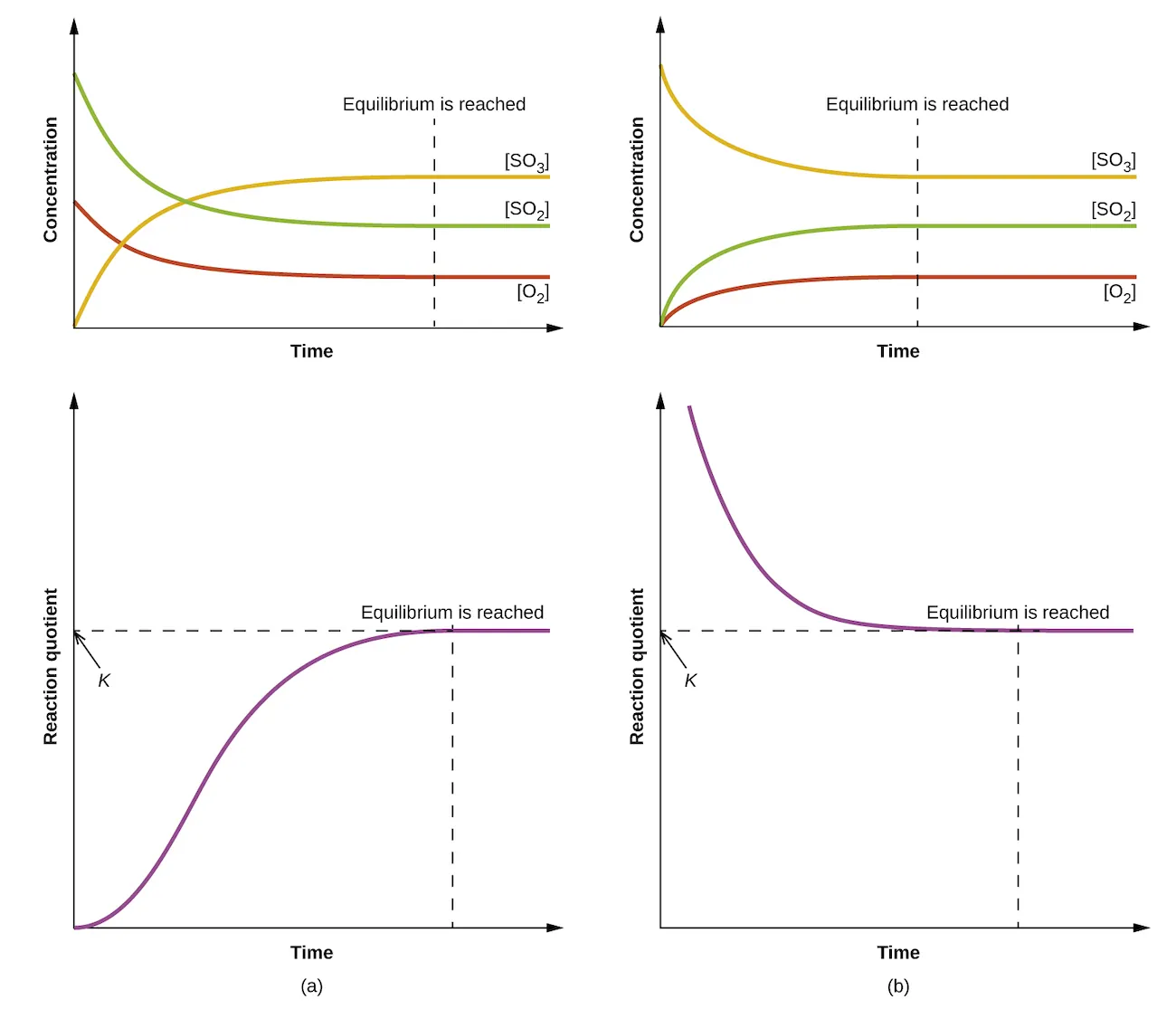
These graphs show concentrations changing until equilibrium is reached, after which the values remain constant. This supports K calculations by emphasising that Kc/Kp must be formed from equilibrium quantities. Extra detail: the figure also includes reaction quotient (Qc) trends over time, which is beyond the minimum needed for this subsubtopic. Source
A key OCR requirement is that calculations must avoid quadratic equations. This is achieved by:
Using given equilibrium concentrations, or
Making justified assumptions where changes are small relative to initial amounts.
Units of Kc
The units of Kc depend on the overall stoichiometry of the reaction. They are not always unitless.
Determining Kc units
To find units:
Multiply the units of all product concentration terms.
Divide by the units of all reactant concentration terms.
Cancel where possible.
Important points:
Concentration units are mol dm⁻³.
Units depend on the difference between total powers of products and reactants.
If powers are equal, units cancel, making Kc unitless.
For example, if there are more moles of gaseous products than reactants, Kc will have units involving mol dm⁻³ raised to a positive power.
Calculating Kp from Equilibrium Data
Kp is used for equilibria involving gases, where quantities are expressed as partial pressures rather than concentrations.

This diagram illustrates Dalton’s law of partial pressures, where the total pressure of a gas mixture equals the sum of the component partial pressures. It connects the idea of separate gas contributions to the partial pressures used directly in a Kp expression. Source
Kp expression and calculation principles
Kp calculations follow the same structural logic as Kc but replace concentration with partial pressure.
Equilibrium constant (Kp): The ratio of the equilibrium partial pressures of gaseous products to reactants, each raised to the power of their stoichiometric coefficients.
Only gaseous species appear in a Kp expression. Solids and liquids are omitted entirely, as their pressures are constant.
To calculate Kp:
Write the balanced equation.
Write the Kp expression using partial pressures.
Substitute equilibrium partial pressures.
Evaluate the numerical value.
Units of Kp
Kp units depend on the pressure units used and the stoichiometry of the gaseous species.
Determining Kp units
Partial pressure is commonly measured in kPa.
Units are derived by comparing total powers of products and reactants.
If powers are equal, Kp has no units.
If the number of moles of gaseous products differs from gaseous reactants, Kp will carry units such as kPa or kPa⁻¹.
Students must always state units unless the value is dimensionless.
Relationship between Kc and Kp
Kc and Kp are related for gaseous equilibria through temperature and the gas constant.
Relationship between Kp and Kc
Kp = Kc(RT)ⁿ
n = Difference between total moles of gaseous products and reactants
R = Gas constant (8.31 J mol⁻¹ K⁻¹)
T = Temperature in kelvin (K)
This relationship allows conversion between Kc and Kp when required data are provided. Temperature must always be in kelvin.
A sentence must separate this equation from any other definition or equation, ensuring clarity and compliance with formatting rules.
Avoiding Quadratic Equations
The specification explicitly states that Kc and Kp calculations should be carried out without using quadratic equations.
This is achieved by:
Using equilibrium values directly.
Applying valid approximations where changes are small.
Selecting data where equilibrium amounts are already provided.
Students are expected to recognise when simplifying assumptions are appropriate and when they are not required.
Common Pitfalls and OCR Expectations
OCR assessments expect:
Correct equilibrium expressions based on balanced equations.
Accurate substitution of equilibrium values only.
Correct identification and calculation of units.
Clear distinction between Kc and Kp.
Use of kelvin for temperature where relevant.
Key errors to avoid include:
Using initial values instead of equilibrium values.
Including solids or liquids in Kc or Kp expressions.
Omitting units where they do not cancel.
Mixing concentration and pressure data incorrectly.
Understanding how to calculate Kc and Kp, and correctly assign units, is essential for analysing equilibrium systems and forms a foundation for more advanced equilibrium concepts later in the course.
FAQ
Kc itself does not change at constant temperature. Apparent changes usually arise from using incorrect values in the calculation.
Common causes include:
Using initial rather than equilibrium concentrations
Rounding values too early
Incorrect stoichiometric powers in the Kc expression
Careful use of equilibrium data and correct algebra ensures a consistent Kc value.
Changing volume alters the partial pressures of gases, which shifts the equilibrium position, but does not change the value of Kp at constant temperature.
However, volume changes:
Modify equilibrium partial pressures
Affect calculated reaction quotients (Qp)
Require recalculation of equilibrium pressures before Kp is evaluated
Kp must always be calculated using equilibrium pressures after the system has re-established equilibrium.
The magnitude of Kc or Kp reflects how far a reaction lies towards products or reactants at equilibrium.
Very large values indicate equilibrium lies strongly to the right
Very small values indicate equilibrium lies strongly to the left
This behaviour is influenced by reaction energetics and temperature, not by initial concentrations or pressures.
The relationship between Kc and Kp includes the gas constant R, which is defined using absolute temperature.
Using kelvin:
Ensures consistency with thermodynamic equations
Prevents mathematically invalid negative or zero temperatures
Produces physically meaningful values for Kp
Using Celsius would give incorrect results.
Kp calculations often require fewer steps because partial pressures can be obtained directly from total pressure and mole fractions.
This avoids:
Converting moles to concentrations
Accounting for changing volume explicitly
As a result, Kp is often preferred for gas-phase equilibria, especially when pressure data are given directly.
Practice Questions
A reversible reaction reaches equilibrium at a constant temperature. Explain why the value of Kc for this reaction has no units.
(2 marks)
States that the powers of the concentration terms for products and reactants are equal, so the units cancel. (1 mark)
Recognises that Kc is unitless when the total moles of products equal the total moles of reactants in the equilibrium expression. (1 mark)
A reversible reaction occurs in the gas phase:
2SO₂(g) + O₂(g) ⇌ 2SO₃(g)
At equilibrium, the partial pressures of the gases are known.
(a) Write the correct expression for Kp for this reaction.
(b) State the units of Kp and explain your reasoning.
(c) State one reason why Kp cannot be calculated using concentrations for this reaction.
(5 marks)
(a) Kp expression (2 marks)
Correct form of expression: Kp = (p(SO₃))² / (p(SO₂))² p(O₂). (2 marks)
1 mark if expression structure is correct but powers are incorrect or missing.
(b) Units of Kp (2 marks)
States correct units: kPa⁻¹. (1 mark)
Explains units arise because there are fewer moles of gaseous products than reactants, so pressure units do not cancel. (1 mark)
(c) Use of concentrations (1 mark)
States that Kp is defined using partial pressures for gases and concentrations are used only for Kc. (1 mark)

