OCR Specification focus:
‘Use ionic equations to show H⁺ role in reactions with metals, carbonates, metal oxides and alkalis.’
Acids react in characteristic ways with metals, bases, oxides, and carbonates, and these reactions can be clearly represented using ionic equations showing the role of hydrogen ions.
Nature of Acid Reactions
Acids are substances that produce hydrogen ions, H⁺(aq) when dissolved in water. In aqueous reactions, it is these hydrogen ions that are chemically active, not the acid molecules themselves. Writing reactions in ionic form helps highlight this role and removes spectator ions that do not participate in the chemical change.
Many reactions of acids are examples of neutralisation, where acidic hydrogen ions react with basic species to form water, or redox reactions, particularly when acids react with metals.
Reactions of Acids with Metals
When an acid reacts with a reactive metal, a salt and hydrogen gas are produced. The metal atoms lose electrons and are oxidised, while hydrogen ions gain electrons and are reduced.
The general reaction is:
Acid + Metal → Salt + Hydrogen
Only metals above hydrogen in the reactivity series will react readily with dilute acids. Common examples include magnesium, zinc, and iron reacting with dilute hydrochloric or sulfuric acid.
The key feature is the reduction of hydrogen ions to hydrogen gas.
Oxidation: Loss of electrons.
Reduction: Gain of electrons.
When a reactive metal is added to a dilute acid, H₂(g) is released (seen as effervescence) and an aqueous salt is formed.

Magnesium reacts with hydrochloric acid, producing visible bubbles of hydrogen gas as the metal dissolves to form a magnesium salt in solution. Source
In ionic terms, the reaction can be simplified by removing spectator ions such as Cl⁻ or SO₄²⁻. This shows the essential chemistry clearly.
Acid–metal reaction (ionic)
Mg(s) + 2H⁺(aq) → Mg²⁺(aq) + H₂(g)
H⁺ = hydrogen ion, aqueous
Mg²⁺ = magnesium ion, aqueous
This ionic equation applies to all acid–metal reactions where hydrogen gas is evolved, regardless of the specific acid used.
Reactions of Acids with Bases and Alkalis
Bases are substances that neutralise acids. Alkalis are soluble bases that release hydroxide ions, OH⁻(aq), in solution. When acids react with bases or alkalis, the defining process is neutralisation.
The general reaction is:
Acid + Base → Salt + Water
In aqueous solution, the key reacting species are hydrogen ions and hydroxide ions.
Neutralisation: A reaction in which hydrogen ions react with hydroxide ions to form water.
The ionic equation for all strong acid–strong alkali reactions is identical, regardless of the substances used.
Neutralisation (ionic)
H⁺(aq) + OH⁻(aq) → H₂O(l)
OH⁻ = hydroxide ion, aqueous
In reactions with alkalis, the key chemical change is neutralisation, where H⁺(aq) reacts with OH⁻(aq) to form H₂O(l).

The images show bromothymol blue changing colour as alkali is added and hydrogen ions are neutralised by hydroxide ions. The specific example shown involves ascorbic acid, which goes beyond the syllabus focus but clearly illustrates neutralisation. Source
This equation demonstrates that the formation of water is the central chemical change, with all other ions acting as spectators.
Reactions of Acids with Metal Oxides
Metal oxides are basic and react with acids in neutralisation reactions to form a salt and water. These reactions are not redox processes; instead, they involve acid–base chemistry.
The oxide ion, O²⁻, is a strong base and reacts with hydrogen ions from the acid.
In molecular terms:
Acid + Metal oxide → Salt + Water
When written ionically, the reaction focuses on hydrogen ions reacting with oxide ions.
Acid–metal oxide reaction (ionic)
O²⁻(aq) + 2H⁺(aq) → H₂O(l)
O²⁻ = oxide ion
This ionic equation shows that two hydrogen ions are required to neutralise each oxide ion, forming water. Solid metal oxides must first dissolve or react at the surface for the ions to participate.
Reactions of Acids with Metal Hydroxides
Metal hydroxides, whether soluble or insoluble, also react with acids via neutralisation. The reactive species is the hydroxide ion.
Examples include reactions of acids with sodium hydroxide, calcium hydroxide, or insoluble hydroxides such as copper(II) hydroxide.
The essential ionic process is identical to that of alkalis.
Hydrogen ions react with hydroxide ions to form water.
The remaining ions form the salt.
This reinforces that neutralisation reactions always involve H⁺ and OH⁻ ions, regardless of solubility.
Reactions of Acids with Carbonates
Carbonates and hydrogencarbonates react with acids to produce carbon dioxide, water, and a salt. These reactions are useful tests for carbonates due to the effervescence of CO₂.
The general reaction is:
Acid + Carbonate → Salt + Water + Carbon dioxide
The chemistry involves hydrogen ions reacting with carbonate ions, CO₃²⁻.
Carbonates react with acids to produce an aqueous salt, water, and carbon dioxide gas, so you observe strong effervescence.
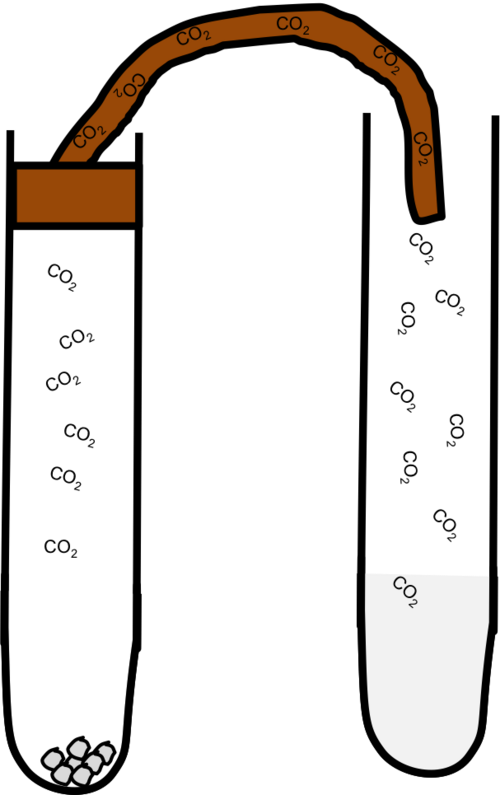
The diagram shows hydrogen ions reacting with carbonate ions to form carbon dioxide and water. The limewater test shown is additional detail beyond the core syllabus but helps confirm the identity of the gas produced. Source
Acid–carbonate reaction (ionic)
CO₃²⁻(aq) + 2H⁺(aq) → CO₂(g) + H₂O(l)
CO₃²⁻ = carbonate ion
This equation shows that two hydrogen ions are needed to fully react with each carbonate ion. The formation of a gas drives the reaction to completion.
Importance of Ionic Equations
Using ionic equations:
Highlights the role of hydrogen ions in acid reactions
Removes irrelevant spectator ions
Allows comparison of reactions involving different acids
Reinforces understanding of acids as H⁺ donors in aqueous solution
Across all these reactions, the defining chemical behaviour of acids is consistently shown by the involvement of hydrogen ions reacting with metals, bases, oxides, hydroxides, or carbonates.
FAQ
The rate of reaction depends not only on position in the reactivity series but also on physical and chemical factors.
These include:
Formation of an insoluble coating (e.g. aluminium oxide) that prevents contact with the acid
Surface area of the metal
Concentration of hydrogen ions in the acid
These factors affect how easily hydrogen ions can gain electrons from the metal.
Copper and silver are below hydrogen in the reactivity series.
This means:
Their atoms do not readily lose electrons to hydrogen ions
Hydrogen ions cannot be reduced to hydrogen gas
As a result, no redox reaction occurs between dilute acids and these metals.
A metal oxide contains the oxide ion, O²⁻, which has a 2− charge.
Each oxide ion:
Requires two hydrogen ions to fully neutralise its charge
Forms one water molecule when neutralised
This explains why oxide ions always react with acids in a 1:2 ratio.
Carbonate ions form an unstable intermediate, carbonic acid, when they react with hydrogen ions.
Carbonic acid:
Quickly decomposes into carbon dioxide and water
Makes the reaction irreversible due to gas escape
This gas evolution is responsible for the vigorous effervescence observed.
Ionic equations show only the species directly involved in the chemical change.
They:
Emphasise the role of hydrogen ions
Remove spectator ions that do not affect the reaction
Allow different acids to be compared using the same equation
This clarity is essential for demonstrating understanding at A-Level.
Practice Questions
Dilute hydrochloric acid reacts with excess magnesium ribbon.
a) State the gas produced in this reaction.
b) Write the ionic equation for this reaction.
(2 marks)
a) Hydrogen gas
1 mark for correctly stating hydrogen
b) Mg(s) + 2H⁺(aq) → Mg²⁺(aq) + H₂(g)
1 mark for correct ionic equation showing magnesium reacting with hydrogen ions
Hydrochloric acid reacts separately with sodium hydroxide solution and with calcium carbonate.
a) Write the ionic equation for the reaction between hydrochloric acid and sodium hydroxide.
b) Write the ionic equation for the reaction between hydrochloric acid and calcium carbonate.
c) Explain, in terms of ions, why effervescence is observed when hydrochloric acid reacts with calcium carbonate.
(5 marks)
a) H⁺(aq) + OH⁻(aq) → H₂O(l)
1 mark for correct ionic equation for neutralisation
b) CO₃²⁻(aq) + 2H⁺(aq) → CO₂(g) + H₂O(l)
2 marks
1 mark for correct reactants with correct charges
1 mark for correct products (carbon dioxide and water)
c) Explanation of effervescence
2 marks
1 mark for stating that hydrogen ions react with carbonate ions
1 mark for stating that carbon dioxide gas is formed, causing bubbling

