OCR Specification focus:
‘Calculate pH for strong acids and bases (using Kw), and for weak acids using appropriate approximations.’
These notes explain how to calculate pH for strong acids, strong bases, and weak acids, focusing on assumptions, equilibrium ideas, and syllabus-specific chemical reasoning.
Understanding pH and Hydrogen Ion Concentration
pH calculations are central to acid–base chemistry because they quantify acidity and alkalinity using hydrogen ion concentration. All pH calculations ultimately depend on the relationship between [H⁺], [OH⁻], and water equilibrium.
pH is first introduced as a logarithmic measure of hydrogen ion concentration, allowing very large concentration ranges to be handled conveniently.
pH: A logarithmic measure of hydrogen ion concentration defined as −log₁₀[H⁺], where [H⁺] is in mol dm⁻³.
The logarithmic nature of pH means that small numerical changes correspond to large chemical differences. Accurate identification of [H⁺] is therefore essential before calculating pH.
Strong Acids and pH Calculations
Strong acids are characterised by complete dissociation in aqueous solution. This means that every acid molecule donates a proton to water, making pH calculations relatively direct.
Strong acid: An acid that fully dissociates in aqueous solution to produce hydrogen ions.
For a monoprotic strong acid, the hydrogen ion concentration equals the acid concentration because dissociation is complete. No equilibrium expression is required.
For strong acids and strong bases, assume complete dissociation, so the acid or base concentration directly gives [H⁺] or [OH⁻].
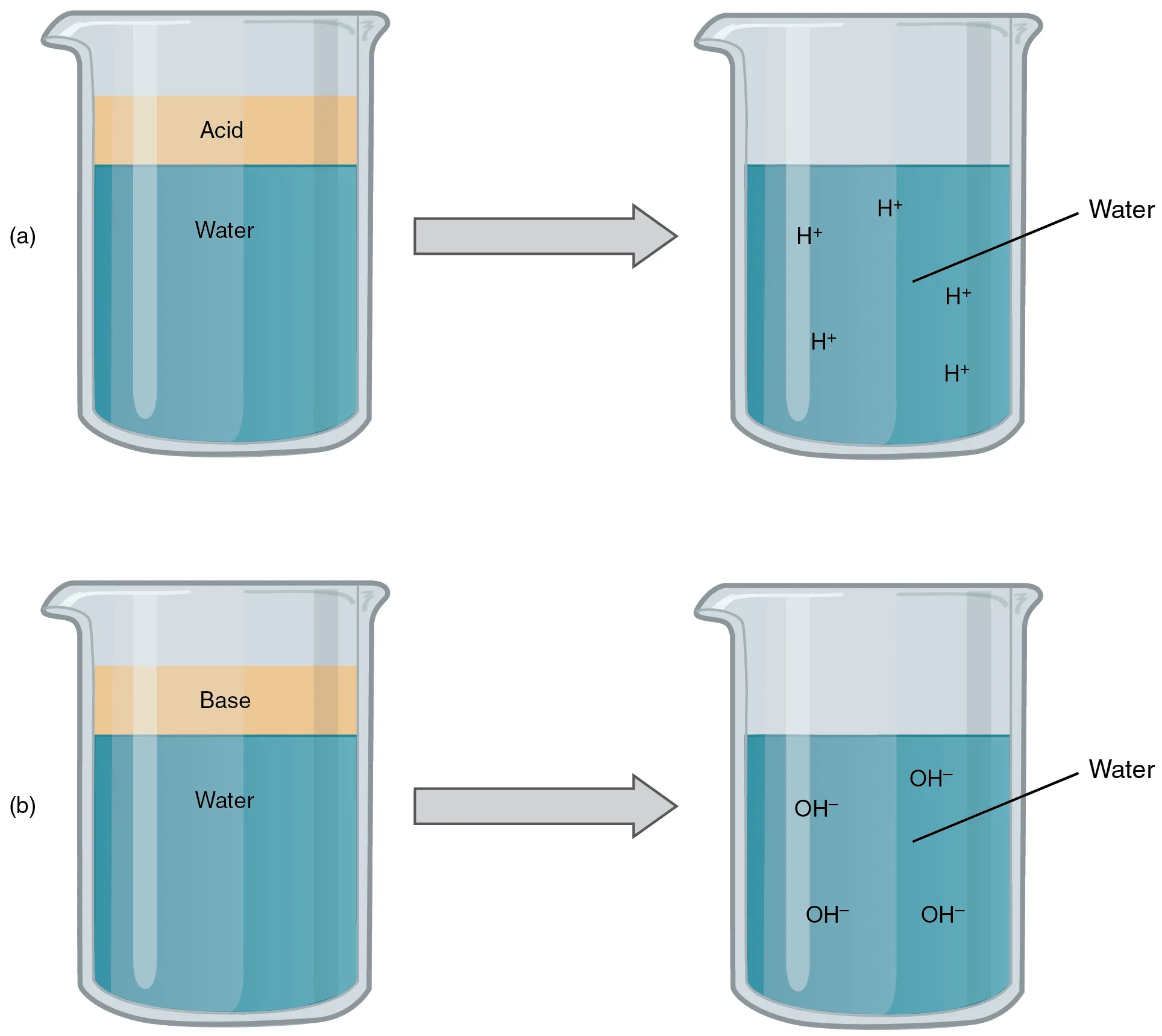
This diagram shows that a strong acid dissociates almost completely to give a high concentration of H⁺, while a strong base dissociates almost completely to give a high concentration of OH⁻. This supports the direct approach used for strong acid and strong base pH calculations. Source
Important features when calculating pH of strong acids include:
Complete ionisation, so no equilibrium assumption is needed
Stoichiometric relationships determine [H⁺]
Water auto-ionisation is negligible compared with acid contribution
When dealing with polyprotic strong acids, the number of protons released per molecule must be considered, as this directly affects [H⁺].
Strong Bases and the Use of Kw
Strong bases fully dissociate to produce hydroxide ions rather than hydrogen ions. Because pH depends on [H⁺], the ionic product of water must be used.
Strong base: A base that fully dissociates in aqueous solution to produce hydroxide ions.
The link between hydroxide ions and hydrogen ions is defined by the ionic product of water.
Equation Name (Kw) = [H⁺][OH⁻]
[H⁺] = hydrogen ion concentration (mol dm⁻³)
[OH⁻] = hydroxide ion concentration (mol dm⁻³)
Kw = 1.0 × 10⁻¹⁴ at 298 K
Kw links [H⁺] and [OH⁻] in all aqueous solutions, because water establishes an equilibrium between H₃O⁺ and OH⁻.
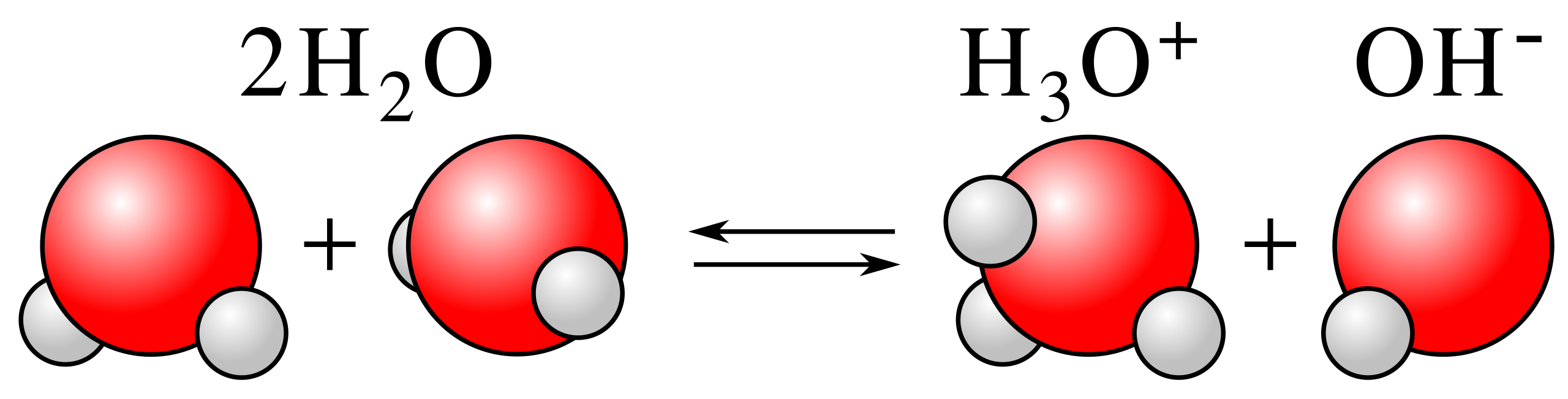
This diagram illustrates the self-ionisation of water, forming hydronium and hydroxide ions. It explains why Kw can be used to convert between [OH⁻] and [H⁺] when calculating pH for strong bases. Source
Using Kw allows conversion between [OH⁻] and [H⁺]. This step is essential before applying the pH definition. Temperature dependence of Kw is not required here, as calculations assume standard conditions unless stated otherwise.
Key points for strong base calculations:
Determine [OH⁻] from base dissociation
Use Kw to calculate [H⁺]
Apply the pH definition only after finding [H⁺]
Weak Acids and Equilibrium Considerations
Weak acids only partially dissociate in aqueous solution, so equilibrium ideas must be applied. Unlike strong acids, hydrogen ion concentration is not equal to the initial acid concentration.
Weak acid: An acid that partially dissociates in aqueous solution, establishing an equilibrium between acid molecules and ions.
Weak acid dissociation is described using an equilibrium constant, Ka, which measures the extent of ionisation.
Equation Name (Ka) = ([H⁺][A⁻]) / [HA]
[H⁺] = hydrogen ion concentration (mol dm⁻³)
[A⁻] = conjugate base concentration (mol dm⁻³)
[HA] = undissociated acid concentration (mol dm⁻³)
Weak acids only partially ionise, so pH is found from an equilibrium using Ka rather than assuming [H⁺] equals the initial acid concentration.
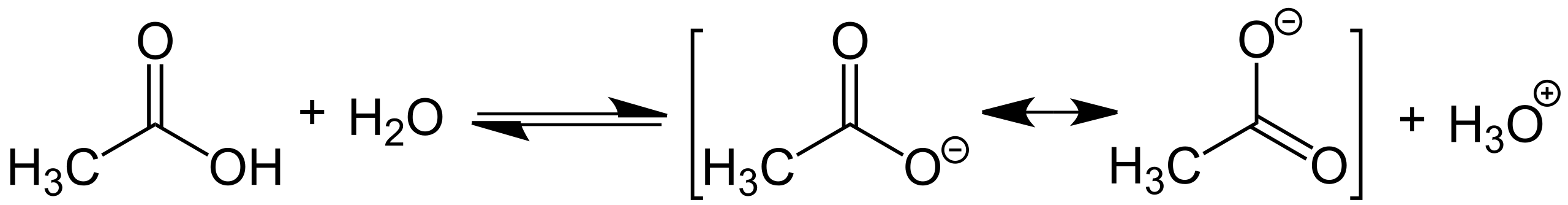
This figure shows a weak acid establishing an equilibrium with water. Although acetic acid is used as the example, it represents the general HA ⇌ H⁺ + A⁻ behaviour applied in weak-acid pH calculations. Source
Approximations in Weak Acid pH Calculations
For many weak acids, dissociation is minimal. This allows simplifying assumptions that make pH calculations manageable without solving complex equations.
The common approximation used is:
[HA] ≈ initial acid concentration
[H⁺] ≈ [A⁻]
These assumptions are valid when the acid is weak and the equilibrium lies far to the left. They allow Ka expressions to be rearranged to estimate [H⁺] directly.
Conditions supporting valid approximations include:
Small Ka values
Relatively high initial acid concentrations
Minimal percentage dissociation
The specification requires recognising and using these approximations appropriately, without extending into situations where they break down.
Comparing Strong and Weak Acid Calculations
Strong and weak acid pH calculations differ fundamentally in approach, even when starting concentrations appear similar.
Key contrasts include:
Strong acids: complete dissociation, no equilibrium calculation
Weak acids: partial dissociation, Ka expression required
Hydrogen ion source: direct for strong acids, equilibrium-controlled for weak acids
Understanding these differences ensures correct method selection, which is essential for accurate pH determination at A-Level standard.
Linking pH Calculations to Chemical Reasoning
pH calculations are not purely mathematical; they reflect underlying chemical behaviour. Recognising whether an acid or base is strong or weak determines whether equilibrium reasoning or direct stoichiometry is appropriate.
Throughout this topic, careful identification of:
Degree of dissociation
Relevant equilibrium constants
Valid approximations
ensures that pH calculations remain chemically meaningful and fully aligned with OCR A-Level Chemistry expectations.
FAQ
In solutions of strong acids, the concentration of hydrogen ions produced by complete dissociation is much larger than that produced by water’s self-ionisation.
The [H⁺] from water (about 1.0 × 10⁻7 mol dm−3 at 298 K) is negligible compared with typical strong acid concentrations, so including it would not significantly change the calculated pH.
The approximation fails when the acid is relatively strong or very dilute.
This occurs when:
Ka is not small compared with the initial acid concentration
A significant proportion of the acid dissociates
In these cases, assuming [HA] remains constant leads to noticeable errors in pH.
Strong bases produce hydroxide ions, not hydrogen ions.
Because pH is defined in terms of [H⁺], the ionic product of water, Kw, must be used to convert [OH⁻] into [H⁺] before applying the pH equation.
Higher initial acid concentrations usually improve the validity of approximations.
This is because only a small fraction of acid molecules dissociate, keeping changes in [HA] minimal and maintaining equilibrium assumptions used in Ka-based calculations.
pH depends on the extent of dissociation, not just concentration.
A strong acid fully dissociates, producing a high [H⁺], whereas a weak acid only partially dissociates, resulting in a lower [H⁺] and higher pH despite equal starting concentrations.
Practice Questions
A solution contains a strong monoprotic acid at a concentration of 0.010 mol dm−3.
a) State the hydrogen ion concentration, [H⁺], in this solution.
b) Calculate the pH of the solution.
(2 marks)
a)
Correctly states [H⁺] = 0.010 mol dm−3 (1 mark)
b)
Correct use of pH = −log[H⁺] giving pH = 2.00 (1 mark)
Ethanoic acid is a weak acid with an acid dissociation constant, Ka, of 1.8 × 10−5 at 298 K. A solution of ethanoic acid has a concentration of 0.20 mol dm−3.
a) Write the equilibrium expression for Ka for ethanoic acid.
b) Explain why an approximation can be used when calculating the pH of this solution.
c) Calculate the pH of the ethanoic acid solution, stating any assumptions made.
(5 marks)
a)
Correct Ka expression: Ka = [H⁺][CH₃COO⁻] / [CH₃COOH] (1 mark)
b)
States that ethanoic acid is weak and only partially dissociates (1 mark)
States that [CH₃COOH] remains approximately equal to the initial concentration or that dissociation is small compared with initial concentration (1 mark)
c)
Correct substitution into Ka expression using the approximation (1 mark)
Correct calculation leading to pH ≈ 2.72 (allow 2.70–2.75) (1 mark)

