OCR Specification focus:
‘Define Brønsted–Lowry acids and bases; identify conjugate acid–base pairs and mono-, di-, tribasic acids.’
These notes explain Brønsted–Lowry acid–base theory, focusing on proton transfer, conjugate pairs, and acid basicity, providing precise definitions and clear chemical context for A-Level study.
Brønsted–Lowry Theory Overview
Brønsted–Lowry theory provides a unifying framework for understanding acid–base reactions in aqueous and non-aqueous systems. The central idea is proton transfer, allowing acids and bases to be identified by their behaviour rather than by composition alone. This approach explains a wide range of reactions, including those where no hydroxide ions are present, and is therefore more general than earlier definitions.
Under this theory, every acid–base reaction involves two substances interacting as a conjugate acid–base pair, linked by the transfer of a single proton, H⁺. This concept is essential for analysing reversible reactions and understanding acid strength.
Brønsted–Lowry Acids and Bases
A Brønsted–Lowry acid and base are defined strictly by their role in proton transfer. This definition applies equally to simple mineral acids, weak organic acids, and species such as ammonia.
Brønsted–Lowry acid: A species that donates a proton (H⁺) to another species.
A Brønsted–Lowry acid must contain hydrogen and be able to release it as a proton. Acids can be neutral molecules or charged ions, and their ability to donate protons varies depending on bond polarity and stability of the products formed.
Brønsted–Lowry base: A species that accepts a proton (H⁺) from another species.
Bases must have a lone pair of electrons or a region of high electron density capable of forming a coordinate bond with H⁺. Like acids, bases may be neutral molecules or negatively charged ions.
In any Brønsted–Lowry reaction, both an acid and a base must be present, as proton donation cannot occur without proton acceptance.
Proton Transfer and Reaction Direction
When an acid donates a proton, it is converted into a new species. Similarly, when a base accepts a proton, it also forms a new species. These resulting species are not arbitrary but are directly related to the original reactants.
Acid–base reactions are often reversible, with the position of equilibrium determined by the relative strengths of the acids and bases involved. Stronger acids favour proton donation, while weaker acids are more likely to retain their proton.
Understanding proton transfer allows prediction of reaction direction and identification of the dominant acid–base pair in equilibrium systems.
Conjugate Acid–Base Pairs
Every Brønsted–Lowry acid–base reaction involves conjugate pairs differing by one proton. Recognising these pairs is a core requirement of the specification.
Conjugate acid–base pair: Two species that differ by one proton (H⁺), formed when an acid donates a proton or a base accepts one.
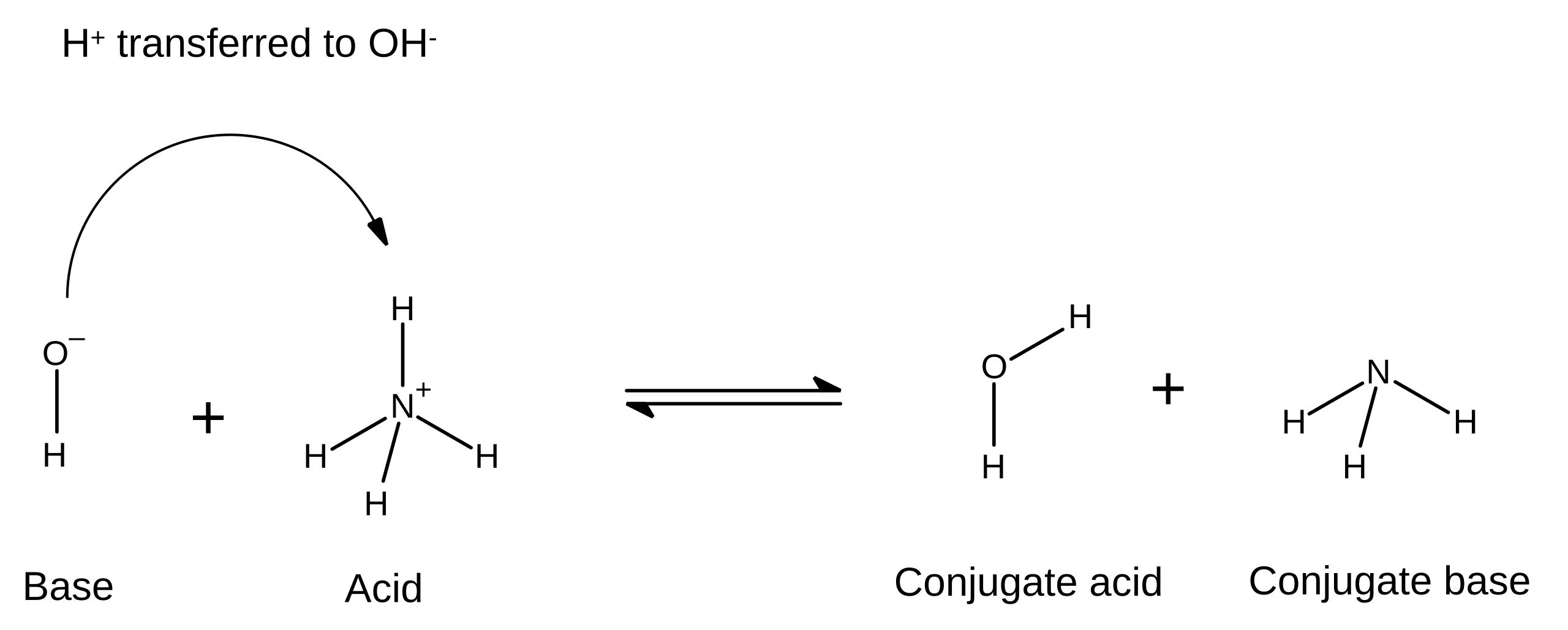
This diagram shows NH₄⁺ donating a proton to OH⁻, forming NH₃ and H₂O. The conjugate pairs are NH₄⁺/NH₃ and H₂O/OH⁻, each differing by one proton. Source
Key features of conjugate pairs include:
The acid forms its conjugate base after losing H⁺
The base forms its conjugate acid after gaining H⁺
Conjugate pairs are always linked within the same reaction
A stronger acid has a weaker conjugate base, because it more readily loses its proton. This inverse relationship is essential for understanding acid strength trends and equilibria.
Amphoteric Behaviour
Some species can act as either an acid or a base depending on the reaction conditions. These are described as amphoteric under Brønsted–Lowry theory.
For example:
Acting as an acid by donating H⁺ to a stronger base
Acting as a base by accepting H⁺ from a stronger acid
This behaviour highlights the importance of relative strength rather than fixed classification.
Basicity of Acids: Mono-, Di-, and Tribasic Acids
Acids can be classified by the number of protons they are capable of donating per molecule. This is known as basicity and is independent of acid strength.
Monobasic acid: An acid that can donate one proton per molecule.
A monobasic acid contains only one ionisable hydrogen capable of proton donation in acid–base reactions.
Dibasic acid: An acid that can donate two protons per molecule, usually in separate steps.
Dibasic acids undergo proton donation in stages, forming intermediate conjugate species. Each proton may be lost with different ease.
Tribasic acid: An acid that can donate three protons per molecule, typically stepwise.
Tribasic acids exhibit multiple dissociation steps, each producing a different conjugate base. The extent of proton loss depends on equilibrium conditions.
Important points regarding basicity:
Basicity depends on the number of transferable protons, not acid strength
Proton donation usually occurs stepwise, not simultaneously
Each dissociation step has its own equilibrium
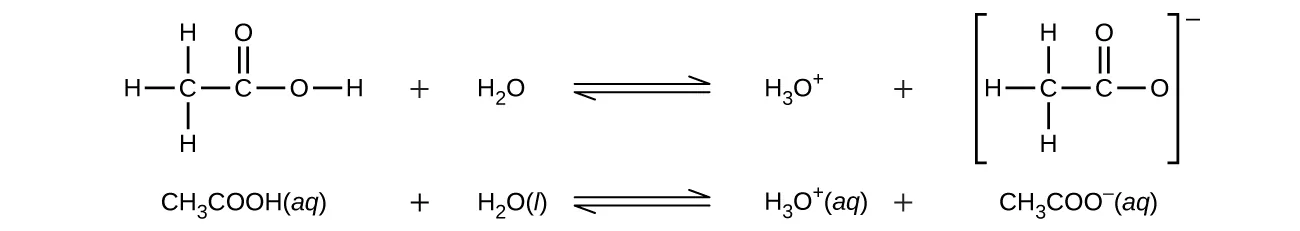
The diagram shows ethanoic acid donating a proton to water to form H₃O⁺ and the conjugate base CH₃COO⁻. This illustrates that ethanoic acid is monobasic because only the –COOH hydrogen is transferred; the explicit hydronium ion formation goes slightly beyond the syllabus but clarifies proton transfer. Source
Distinguishing Strength from Basicity
It is essential not to confuse acid strength with basicity. Strength refers to how readily an acid donates protons, while basicity refers to how many protons it can donate in total. A strong acid may be monobasic, while a weak acid may be dibasic or tribasic.
This distinction is fundamental for correctly interpreting acid–base behaviour within the Brønsted–Lowry framework and for progressing to equilibrium and pH calculations later in the course.
FAQ
The Brønsted–Lowry theory focuses on proton transfer, not the presence of OH− ions.
This allows it to explain reactions such as ammonia reacting with acids, where no hydroxide ions appear. As long as one species donates H+ and another accepts it, the reaction fits the definition, regardless of the solvent used.
The conjugate base is formed when an acid loses one proton.
To identify it:
Start with the acid
Remove one H+
Adjust the charge accordingly
The remaining species is the conjugate base. It will always have one fewer hydrogen than the acid it came from.
Each proton in a polybasic acid is held with a different strength.
After the first proton is lost:
The remaining species is negatively charged
Further proton loss is less favourable due to electrostatic attraction
As a result, each proton is donated in a separate equilibrium step.
Yes. Such species are described as amphoteric under Brønsted–Lowry theory.
Whether they act as an acid or a base depends on the other reactant present. The direction of proton transfer is determined by the relative strengths of the acids and bases involved.
Basicity depends on the number of protons an acid can donate, not how easily it donates them.
An acid may:
Donate one proton very readily (strong, monobasic)
Donate multiple protons less readily (weak, dibasic or tribasic)
This distinction is important when classifying acids and analysing proton transfer reactions.
Practice Questions
Define a Brønsted–Lowry acid and a Brønsted–Lowry base.
(2 marks)
Award one mark for each correct definition.
Brønsted–Lowry acid defined as a proton (H+) donor. (1 mark)
Brønsted–Lowry base defined as a proton (H+) acceptor. (1 mark)
Sulfuric acid, H2SO4, reacts with water according to the Brønsted–Lowry theory.
a) Identify the Brønsted–Lowry acid and Brønsted–Lowry base in the first proton transfer step. (2 marks)
b) State the conjugate acid–base pair formed from sulfuric acid in this step. (2 marks)
c) Explain why sulfuric acid is described as a dibasic acid. (1 mark)
(5 marks)
a) Identification of acid and base (2 marks)
H2SO4 identified as the Brønsted–Lowry acid (proton donor). (1 mark)
H2O identified as the Brønsted–Lowry base (proton acceptor). (1 mark)
b) Conjugate acid–base pair (2 marks)
H2SO4 / HSO4− correctly identified as a conjugate acid–base pair. (1 mark)
Clear indication that the pair differs by one proton. (1 mark)
c) Dibasic explanation (1 mark)
Correct explanation that sulfuric acid can donate two protons per molecule. (1 mark)
No marks awarded for equations or explanations referring to acid strength rather than basicity.

