OCR Specification focus:
‘Recognise when [HA]≈[HA]undissociated and [H+]≈[A−] assumptions become invalid for stronger weak acids.’
Weak-acid pH calculations rely on simplifying assumptions, but these approximations fail under certain conditions, requiring careful chemical reasoning to judge their validity accurately.
Context of Weak-Acid pH Calculations
In aqueous solution, a weak acid only partially dissociates. For an acid HA, an equilibrium is established between undissociated acid molecules and their ions. To simplify pH calculations at A-Level, assumptions are commonly made about the relative sizes of concentrations at equilibrium. These assumptions are valid only when dissociation is small compared to the initial acid concentration.
Understanding the limits of weak-acid approximations is essential, as incorrect use can lead to significant errors in calculated pH values. OCR expects students to recognise situations where approximations break down rather than apply them automatically.
A weak acid only partially dissociates in water, so the equilibrium mixture contains mostly HA with smaller amounts of H⁺ and A⁻.
This diagram shows a weak acid in aqueous solution, where most particles remain as HA and only a small fraction forms H⁺ and A⁻ at equilibrium. The visual supports why weak-acid calculations often assume limited dissociation. Source
The Standard Weak-Acid Approximation
For most weak acids used in introductory calculations, dissociation is minimal. This leads to two linked assumptions that greatly simplify equilibrium expressions.
Weak-acid approximation: The assumption that the equilibrium concentration of undissociated acid is approximately equal to its initial concentration, and that hydrogen ion concentration equals conjugate base concentration.
In practical terms, this means:
[HA] ≈ initial concentration of HA
[H⁺] ≈ [A⁻]
These assumptions allow the Ka expression to be simplified algebraically, avoiding quadratic equations, which are not required by the OCR specification at this level.
Between making these assumptions and calculating pH, it is essential to assess whether the acid behaves as “sufficiently weak” under the given conditions.
Why Approximations Can Fail
Approximations depend on the dissociation being small. If a significant fraction of HA molecules ionise, the difference between initial and equilibrium concentrations becomes non-negligible.
This failure is most likely when:
The acid is relatively strong for a weak acid (larger Ka, smaller pKa)
The initial concentration of the acid is low
Both factors act together
In these cases, assuming that [HA] remains unchanged is chemically inaccurate, and assuming [H⁺] equals [A⁻] no longer reflects the equilibrium composition.
As dissociation becomes more significant, [HA] is no longer effectively ‘unchanged’, and treating [H⁺] as equal to [A⁻] without checks can introduce large errors.
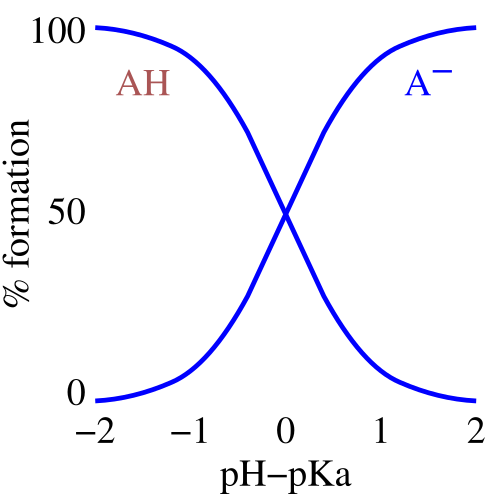
This speciation graph shows how the fraction of HA and A⁻ varies with pH for a generic weak acid system. It highlights that neither species is always dominant, so assumptions such as [HA] ≈ initial concentration can become unreliable under some conditions. Source
Assessing the Size of Dissociation
A key idea underlying this topic is the percentage dissociation of the acid. This describes how much of the acid ionises compared to the amount originally present.
When percentage dissociation is:
Less than about 5%, the approximation is usually acceptable
Greater than about 5%, the approximation becomes unreliable
Although OCR does not require explicit percentage calculations here, students must conceptually recognise when dissociation is too large to ignore.
Always include a clear sentence explaining why the approximation is valid or invalid, based on acid strength and concentration.
Role of Ka and Acid Strength
The acid dissociation constant directly influences whether assumptions hold.
Acid dissociation constant (Ka): A measure of the extent to which an acid dissociates in aqueous solution at equilibrium.
Larger Ka values indicate:
Greater dissociation
Higher equilibrium [H⁺]
Increased likelihood that approximations fail
The stronger the weak acid (larger Ka), the greater the equilibrium [H⁺], so the ‘small x’ approximation is more likely to break down.
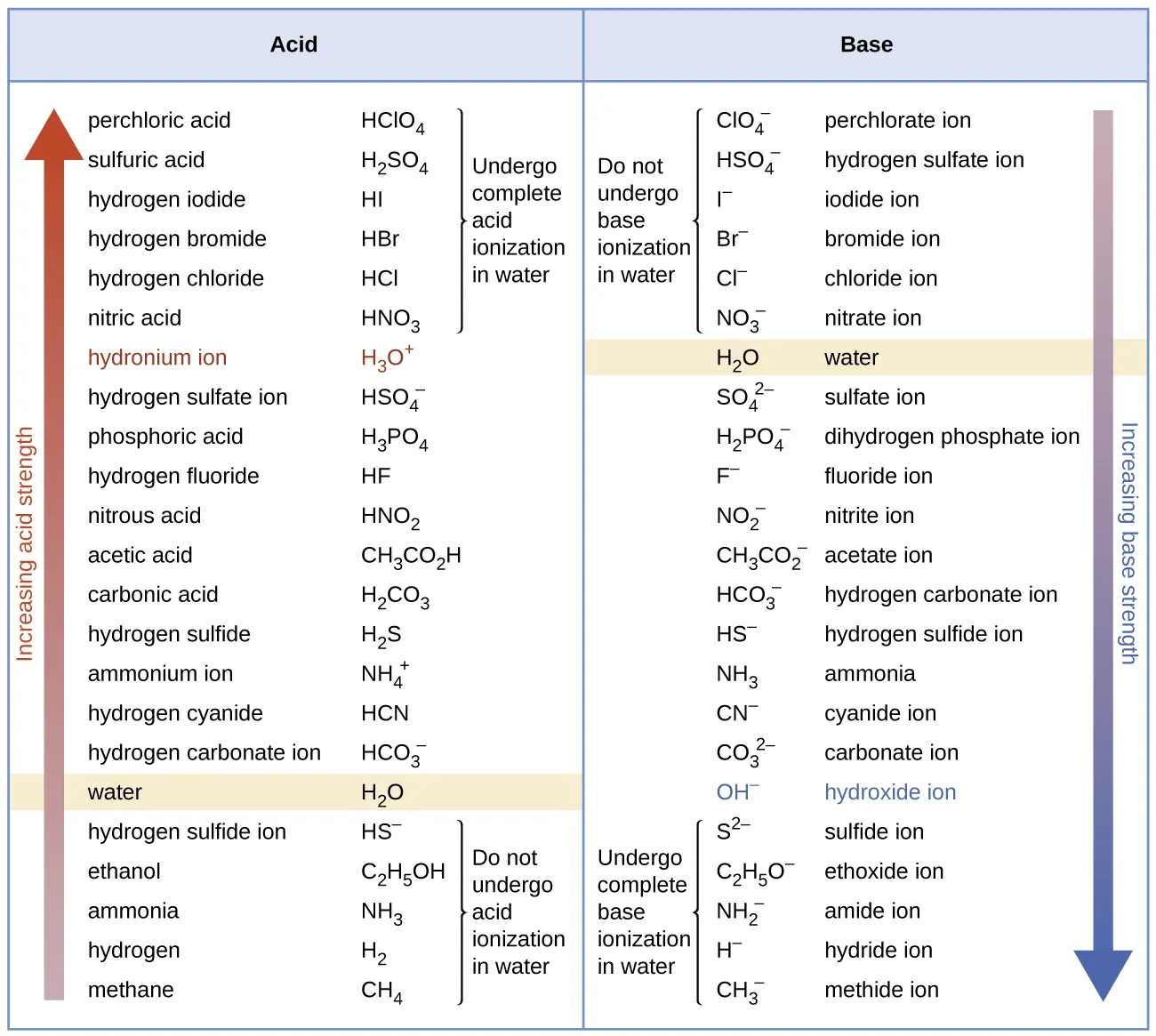
This figure arranges acids by relative strength using Ka values, illustrating why stronger weak acids ionise more extensively. Greater ionisation increases the risk that changes in concentration are no longer negligible in pH calculations. Source
Stronger weak acids, such as those with Ka values close to 10⁻³ or larger, often dissociate too much for simplifications to remain accurate, particularly at low concentrations.
Impact of Initial Acid Concentration
Even a weak acid can show significant dissociation if it is very dilute. At low initial concentrations:
The equilibrium [H⁺] becomes a larger fraction of the starting amount
The change in [HA] cannot be ignored
[H⁺] may no longer equal [A⁻] due to contributions from water
This highlights that acid strength alone is not enough; concentration must also be considered when judging validity.
Identifying Invalid Approximations in Practice
OCR expects recognition, not recalculation, when assumptions fail. Warning signs include:
Ka values that are not “very small”
Stated acid concentrations that are close in magnitude to expected [H⁺]
Questions explicitly asking you to “comment on the validity of the approximation”
In such cases, you should state that:
The assumption [HA] ≈ initial concentration is invalid
The assumption [H⁺] ≈ [A⁻] is invalid
A simplified calculation would underestimate dissociation and miscalculate pH
Relationship to pH Accuracy
When approximations fail:
Calculated pH values are usually too high
True acidity is underestimated
Chemical conclusions based on pH may be incorrect
Recognising these limits reinforces conceptual understanding of equilibrium rather than rote calculation, aligning with OCR’s emphasis on chemical reasoning.
Specification-Focused Summary of Limits
For OCR A-Level Chemistry, students must be able to:
Recognise when weak-acid approximations are invalid
Link failure to acid strength and concentration
Explain why assumptions about [HA] and [H⁺] no longer hold
Avoid applying approximations blindly to stronger weak acids
This knowledge ensures accurate interpretation of acid behaviour and prepares students for more advanced equilibrium analysis later in the course.
FAQ
A good judgement can often be made by comparing the size of Ka with the stated acid concentration.
If the acid has a very small Ka and the concentration is relatively high, dissociation will be minimal and the approximation is usually safe.
Warning signs include Ka values that are not extremely small or concentrations that appear similar in size to typical [H⁺] values, suggesting dissociation may be significant.
When approximations fail, the extent of dissociation is larger than assumed.
This means the actual equilibrium [H⁺] is higher than the calculated value.
As a result, the solution is more acidic than predicted, so the calculated pH is too high.
This systematic error is important when interpreting acidity rather than simply performing calculations.
Yes, temperature can indirectly influence validity.
Increasing temperature generally increases Ka for weak acids, leading to greater dissociation.
Greater dissociation increases the change in [HA] and [H⁺], making approximations less reliable.
Although temperature effects are not usually tested numerically here, they are relevant when reasoning about equilibrium behaviour.
In very dilute solutions, the amount of H⁺ produced by the acid may be similar in magnitude to that produced by water.
This means the assumption that all H⁺ comes from the acid is no longer valid.
As a result, [H⁺] may not equal [A⁻], and ignoring water’s contribution leads to inaccurate pH values.
OCR often rewards chemical reasoning rather than numerical accuracy alone.
Stating when an approximation is invalid shows understanding of equilibrium behaviour.
This can gain credit in explanation or evaluation questions, even when full calculations are not required.
It also helps avoid applying memorised methods in chemically inappropriate situations.
Practice Questions
In weak-acid pH calculations, two common assumptions are often made.
State one condition under which these weak-acid approximations become invalid and explain why the approximation fails in that case.
(2 marks)
1 mark: Correctly states a valid condition, such as:
The weak acid has a relatively large Ka (is a stronger weak acid), or
The initial concentration of the acid is low.
1 mark: Correct explanation, such as:
A significant proportion of the acid dissociates, so the change in [HA] is no longer negligible, or
[H⁺] is no longer equal to [A⁻], making the approximation inaccurate.
A student calculates the pH of a weak acid solution using the assumptions that [HA] ≈ initial concentration of HA and [H⁺] ≈ [A⁻].
Explain why these assumptions may not be valid for stronger weak acids or very dilute solutions. In your answer, refer to acid strength, extent of dissociation, and the effect on the calculated pH.
(5 marks)
1 mark: Recognises that stronger weak acids (larger Ka) dissociate to a greater extent.
1 mark: Explains that greater dissociation means [HA] is no longer approximately equal to its initial concentration.
1 mark: Explains that in dilute solutions, the equilibrium [H⁺] is a significant fraction of the initial acid concentration.
1 mark: States that the assumption [H⁺] ≈ [A⁻] becomes invalid under these conditions.
1 mark: Correctly links invalid assumptions to an incorrect pH value, typically stating that the calculated pH is too high (acidity underestimated).

