OCR Specification focus:
‘A single photon transfers energy to a single surface electron in emission.’
In the photoelectric effect, each photon interacts individually with surface electrons, transferring discrete quanta of energy. This one-to-one interaction illustrates light’s particulate nature.
Photon–Electron Interaction Overview
The photoelectric effect provides strong evidence for the quantum nature of light, showing that electromagnetic radiation consists of discrete packets of energy called photons. Each photon behaves as a single quantum entity that interacts with a single surface electron. The energy transfer during this interaction determines whether the electron can escape the metal surface, leading to photoemission.
When light shines on a metal, photoelectrons are only emitted if the photons have sufficient energy to overcome the metal’s work function — the minimum energy needed for an electron to escape. This observation cannot be explained using classical wave theory, which predicts that increasing light intensity should eventually eject electrons regardless of frequency.
Quantum Nature of Light
Discrete Energy Packets
Classical physics treated light as a continuous wave, suggesting that energy was spread uniformly across the wavefront. However, the photon model proposed by Albert Einstein and based on Planck’s quantum theory states that light’s energy is quantised.
Photon: A quantum of electromagnetic radiation that carries energy proportional to its frequency.
Each photon carries an energy value determined by its frequency (f) according to Planck’s relation.
EQUATION
—-----------------------------------------------------------------
Photon Energy (E) = hf
E = Energy of a photon (joules, J)
h = Planck’s constant (6.63 × 10⁻³⁴ J s)
f = Frequency of radiation (hertz, Hz)
—-----------------------------------------------------------------
This means higher-frequency light (such as ultraviolet) contains higher-energy photons than lower-frequency light (such as visible or infrared).
One-to-One Energy Transfer
The one-to-one photon–electron interaction principle states that each photon interacts with one electron only, transferring all of its energy to that electron.
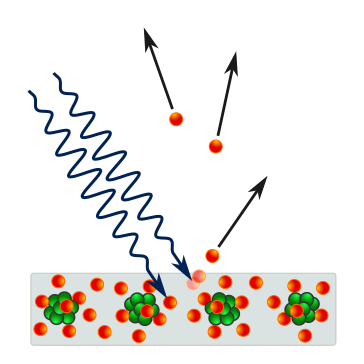
Schematic of the photoelectric effect showing an incident photon causing the emission of a single electron from a crystalline surface. The diagram highlights the discrete, one-to-one transfer of energy required for emission. Labels are minimal and clear to keep focus on the interaction mechanism. Source.
This transfer is instantaneous, supporting the concept that light energy is delivered in discrete quanta rather than continuously.
Key characteristics of this interaction:
A single photon transfers its entire energy to a single surface electron.
If the photon’s energy is less than the work function (Φ) of the material, the electron cannot escape.
If the photon’s energy equals or exceeds the work function, the excess becomes the kinetic energy of the emitted photoelectron.
Increasing the intensity of light increases the number of photons striking the surface but does not change the energy per photon.
This direct, one-to-one correspondence explains why electron emission is frequency dependent and not intensity dependent.
Energy Balance in Photoemission
The energy gained by a surface electron comes from a single photon. If this energy is greater than the work function, the remaining energy manifests as kinetic energy of the photoelectron.
EQUATION
—-----------------------------------------------------------------
Energy Conservation in Photoemission (Einstein’s Photoelectric Equation)
hf = Φ + KEₘₐₓ
hf = Energy supplied by one photon
Φ = Work function, minimum energy required to release an electron (joules, J)
KEₘₐₓ = Maximum kinetic energy of emitted photoelectron (joules, J)
—-----------------------------------------------------------------
This equation illustrates how photon energy divides between overcoming the metal’s binding energy and providing motion to the emitted electron.
Between any two interactions, there is no accumulation of energy from multiple photons. The emission depends solely on the energy of individual photons relative to the work function.
Evidence for the One-to-One Relationship
Experiments on the photoelectric effect demonstrate key results that validate this one-to-one energy transfer:
Immediate emission: Electrons are emitted almost instantaneously when light of sufficient frequency strikes the metal, regardless of intensity. If energy accumulation were gradual, a measurable delay would occur.
Threshold frequency: No electrons are emitted below a specific threshold frequency, confirming that photon energy (hf) must exceed Φ to cause emission.
Intensity dependence: Above the threshold frequency, the rate of photoelectron emission increases with light intensity (more photons), but the maximum kinetic energy remains unchanged.
Linear relationship: The maximum kinetic energy of photoelectrons increases linearly with light frequency, consistent with hf = Φ + KEₘₐₓ.
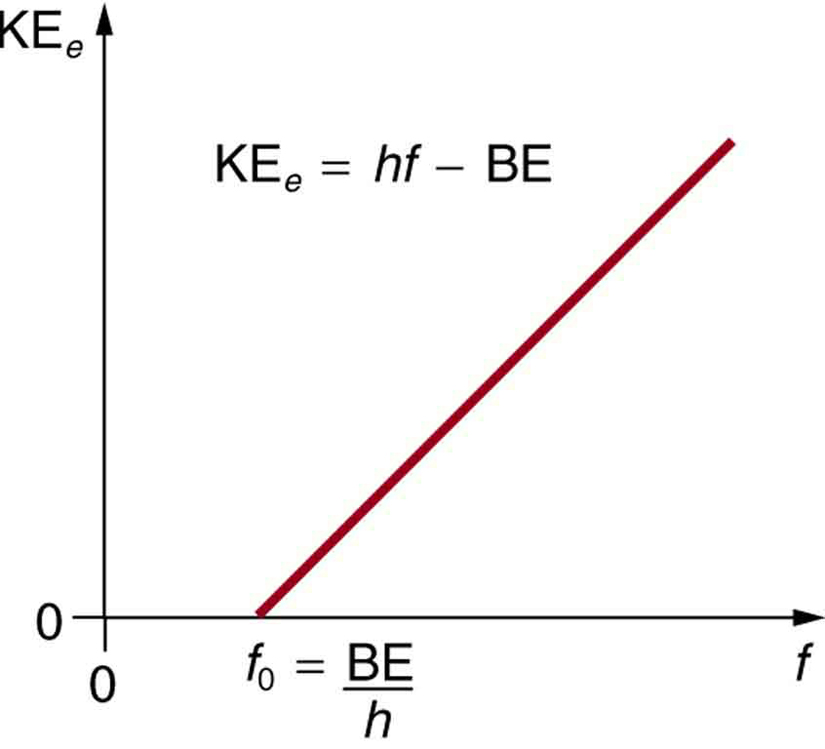
Plot of maximum photoelectron kinetic energy against frequency, intercepting the frequency axis at the threshold frequency. Above threshold, the straight-line slope corresponds to Planck’s constant hhh, consistent with KE,max=hf−ΦK_{E,\max}=hf-\PhiKE,max=hf−Φ. This directly evidences one-to-one photon–electron energy transfer. Source.
These findings conclusively support the photon model of light and the quantum interaction mechanism between photons and electrons.
Microscopic View of the Interaction
On a microscopic scale, the process unfolds as follows:
A photon approaches the metal surface and encounters a surface electron.
The photon’s quantum of energy (hf) is absorbed entirely by that electron.
If the energy exceeds the work function, the electron gains sufficient energy to escape the surface.
The excess energy appears as kinetic energy, determining the electron’s speed once it leaves the metal.
Electrons deeper in the metal usually do not escape because they lose energy through collisions before reaching the surface.
This discrete mechanism contrasts sharply with the continuous energy distribution suggested by wave theory.
Theoretical Implications
The concept of one photon interacting with one electron underpins the development of quantum mechanics. It demonstrates that electromagnetic radiation cannot be understood solely as a wave phenomenon but also exhibits particle-like behaviour.
The photoelectric effect therefore:
Provides direct evidence of energy quantisation in electromagnetic radiation.
Bridges the gap between classical electromagnetism and quantum physics.
Forms the foundation for modern applications such as photodiodes, solar cells, and photomultiplier tubes, which rely on photon–electron interactions for energy conversion.
Summary of Key Points
Each photon transfers energy to one electron only; there is no collective accumulation.
The energy of a photon is given by E = hf, showing its dependence on frequency.
Electron emission occurs only when hf ≥ Φ, leading to measurable photoelectric current.
The instantaneous nature of emission and threshold frequency confirm the quantum description of light.
This subsubtopic directly links to the acceptance of the photon model and the shift from classical to quantum physics.
FAQ
Not all surface electrons are bound equally tightly. The work function refers to the minimum energy required to remove the most loosely bound electrons at the surface.
Electrons deeper in the metal or in different energy states require slightly more energy to escape. Therefore, even though each photon transfers a fixed energy (hf), electrons released from deeper levels have less remaining energy as kinetic energy, producing a range rather than a single value.
Photon energy depends directly on frequency. When frequency increases, the energy of each photon (hf) increases.
Once the work function (Φ) has been overcome, any additional photon energy becomes kinetic energy for the emitted electron. Hence, higher-frequency light gives electrons greater speeds upon emission, resulting in higher maximum kinetic energy.
Work function (Φ): Each material has a unique minimum energy required to release an electron.
Surface cleanliness: Oxidised or contaminated surfaces increase Φ, reducing emission.
Crystal orientation: Slight variations in atomic structure can influence how easily electrons escape.
Metals like caesium and potassium have low work functions, making them ideal for photoemissive surfaces in detectors and photocells.
Visible light photons have relatively low frequencies and therefore insufficient energy (hf) to overcome the typical work function of metals such as zinc or copper.
Only ultraviolet or higher-frequency radiation can supply enough energy per photon to free an electron from these surfaces.
However, metals with low work functions, such as caesium, can emit electrons when exposed to visible light, which is why they are used in light sensors.
In the standard photoelectric effect, each electron absorbs energy from one photon only. The process is strictly one-to-one because photon energy is delivered instantaneously.
However, in extremely intense light sources like lasers, a multi-photon photoelectric effect can occur. This non-linear phenomenon involves an electron absorbing two or more photons simultaneously, though it requires very high photon flux and is not part of the A-Level syllabus.
Practice Questions
Question 1 (2 marks)
Explain what is meant by the term one-to-one photon–electron interaction in the context of the photoelectric effect.
Mark Scheme:
1 mark for stating that each photon transfers its energy to a single electron only.
1 mark for stating that the energy transfer is discrete or quantised, i.e., photons do not share or combine their energies to release an electron.
Question 2 (5 marks)
A clean zinc surface is illuminated with monochromatic ultraviolet light of sufficient frequency to cause photoelectric emission. Describe and explain how the one-to-one photon–electron interaction accounts for the following experimental observations:
(a) Electrons are emitted almost instantaneously after illumination begins.
(b) Increasing the intensity of light increases the number of emitted electrons but not their maximum kinetic energy.
(c) Below a certain frequency of incident light, no electrons are emitted, regardless of intensity.
Mark Scheme:
(a) (1 mark)
Each photon interacts with one surface electron, so energy transfer occurs instantly, resulting in immediate emission when photon energy exceeds the work function.
(b) (2 marks)
Increasing light intensity means more photons per second, leading to more emitted electrons (1 mark).
Each photon’s energy is determined by its frequency, not intensity, so maximum kinetic energy remains unchanged (1 mark).
(c) (2 marks)
Below the threshold frequency, photon energy (hf) is less than the work function (Φ) (1 mark).
Therefore, no single photon can provide enough energy to release an electron, and no emission occurs (1 mark).

