OCR Specification focus:
‘Maximum kinetic energy of photoelectrons is independent of radiation intensity above threshold.’
The photoelectric effect demonstrates how light can eject electrons from a metal surface, providing crucial evidence for the particle nature of light. This subtopic explores how varying light intensity affects the maximum kinetic energy of emitted photoelectrons, revealing the fundamental role of photon energy rather than wave amplitude.
Understanding the Photoelectric Effect
When electromagnetic radiation of sufficiently high frequency strikes a metal surface, electrons are emitted. These electrons are called photoelectrons. The phenomenon cannot be explained by classical wave theory, which predicted that light intensity alone should determine the energy of emitted electrons. Instead, experimental evidence shows that light frequency, not intensity, governs whether emission occurs and the energy of the ejected electrons.
Each electron in the metal can only absorb energy from a single photon — a discrete packet of electromagnetic energy. The amount of energy carried by each photon depends solely on the radiation frequency.
EQUATION
—-----------------------------------------------------------------
Photon Energy (E) = h f
E = Energy of a photon (joules, J)
h = Planck’s constant (6.63 × 10⁻³⁴ J s)
f = Frequency of the incident radiation (hertz, Hz)
—-----------------------------------------------------------------
Thus, for photoelectric emission to occur, the photon energy must exceed the work function of the metal — the minimum energy required to release an electron from the surface.
The Threshold Frequency
Every metal has a characteristic threshold frequency. Below this frequency, no electrons are emitted, regardless of the intensity of the light. This demonstrates that the photoelectric effect is a quantum process dependent on photon energy, not cumulative wave energy.
Threshold frequency: The minimum frequency of electromagnetic radiation required to just cause photoelectric emission from a metal surface.
If the frequency is above the threshold, emission occurs immediately, and the excess photon energy appears as kinetic energy of the emitted electron.
Einstein’s Photoelectric Equation
Einstein proposed an energy conservation relationship for the photoelectric effect that links photon energy, the work function, and the kinetic energy of the emitted electron.
EQUATION
—-----------------------------------------------------------------
Einstein’s Photoelectric Equation: h f = Φ + Kₑ(max)
h f = Energy of incident photon (J)
Φ = Work function of the metal (J)
Kₑ(max) = Maximum kinetic energy of emitted electron (J)
—-----------------------------------------------------------------
This equation shows that the maximum kinetic energy of photoelectrons depends only on the frequency of the incident radiation and the material’s work function — not the intensity.
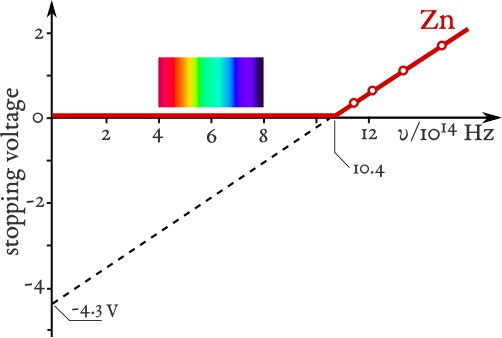
Stopping potential VsV_sVs (proportional to KmaxK_\text{max}Kmax) plotted against frequency for zinc. The linear relation and x-intercept mark the threshold frequency. The labelled metal (Zn) is specific, but the relationship holds for any photoemissive material. Source.
Intensity of Radiation and Its Meaning
In the context of the photoelectric effect, intensity refers to the total energy incident on the metal surface per unit area per second. Increasing intensity means increasing the number of photons striking the surface each second, but each photon retains the same energy determined by its frequency.
Intensity of radiation: The power of electromagnetic radiation incident per unit area, proportional to the number of photons striking the surface per second.
Therefore, intensity influences the rate at which electrons are emitted but not their individual energies.
Independence of Maximum Kinetic Energy from Intensity
Experimental evidence shows that once the threshold frequency is exceeded, increasing the light intensity increases the number of photoelectrons emitted but not their maximum kinetic energy. Each photoelectron receives energy from a single photon; since photon energy depends only on frequency, not on intensity, the kinetic energy remains constant for a given frequency.
Key points:
Photon energy (h f) determines whether emission occurs and the maximum kinetic energy of emitted electrons.
Light intensity affects how many photons strike the surface per second, influencing the emission rate, not the electron energy.
If intensity increases while frequency remains constant (and above threshold), more electrons are emitted per second, but each has the same maximum kinetic energy.
Below the threshold frequency, no electrons are emitted, regardless of how high the intensity is.
This behaviour is consistent with the particle model of light but not with classical wave predictions, which would expect the energy of emitted electrons to increase with light amplitude (intensity).
Experimental Observations
Typical photoelectric experiments involve shining monochromatic light of varying intensity on a metal surface and measuring the stopping potential — the potential difference needed to stop the fastest electrons from reaching the detector. The stopping potential provides a direct measure of the maximum kinetic energy of emitted electrons.
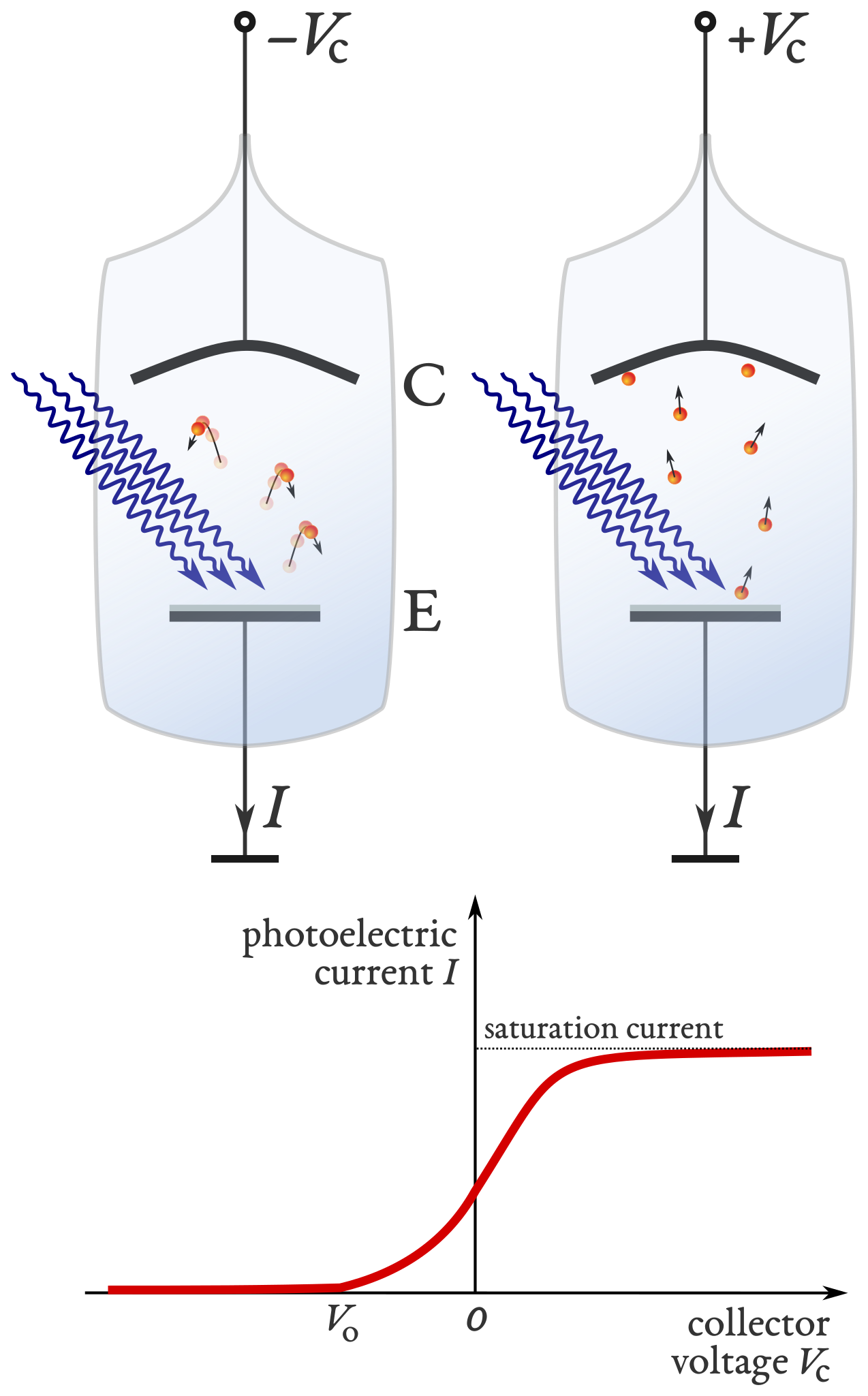
Schematic of a photoelectric tube showing emitter (E), collector (C), and applied potential used to determine the stopping potential. Extra detail: the caption also mentions saturation current (related to high intensity), which goes beyond the OCR requirement but does not add conceptual complexity. Source.
EQUATION
—-----------------------------------------------------------------
Kₑ(max) = e Vₛ
Kₑ(max) = Maximum kinetic energy of emitted electron (J)
e = Elementary charge (1.60 × 10⁻¹⁹ C)
Vₛ = Stopping potential (V)
—-----------------------------------------------------------------
Observations reveal that:
For a given frequency (above threshold), increasing intensity increases current (more photoelectrons) but leaves the stopping potential unchanged.
For higher frequencies, the stopping potential — and hence the maximum kinetic energy — increases, confirming dependence on photon energy.
Conceptual Explanation
The independence of maximum kinetic energy from intensity can be reasoned through one-to-one photon–electron interactions. Each photon interacts with only one electron, transferring its discrete energy quantum. Therefore:
The energy per photon remains constant for a given frequency.
Increasing intensity simply means more photons, so more interactions occur, resulting in higher emission rates but not greater individual energies.
The frequency of the photons sets the energy ceiling for emitted electrons, explaining why maximum kinetic energy remains fixed as intensity changes.
This direct proportionality between photon energy and electron kinetic energy was a pivotal piece of evidence leading to the acceptance of quantum theory in physics. It showed that electromagnetic radiation behaves as if composed of discrete particles — photons — contradicting classical wave models.
Summary of Key Relationships
Photon energy E = h f.
Photoemission requires h f ≥ Φ.
Kₑ(max) = h f – Φ.
Intensity ↑ ⇒ Emission rate ↑ but Kₑ(max) unchanged.
These principles underpin our understanding of the quantum behaviour of light and matter, defining a cornerstone of modern physics.
FAQ
Classical wave theory predicts that increasing light intensity should increase the energy transferred to electrons, as higher intensity means greater wave amplitude and energy.
However, experiments show that the maximum kinetic energy of photoelectrons is unaffected by intensity — it depends only on frequency.
This failure occurs because classical theory assumes energy is distributed continuously across the wavefront, whereas in reality, light energy is quantised into discrete photons. Each photon interacts with one electron, making photon energy (and thus kinetic energy) independent of intensity.
Even with extremely high intensity, no photoelectrons would be emitted.
This is because each photon has energy below the metal’s work function, so individual photons cannot release electrons regardless of their number.
Increased intensity only means more low-energy photons striking the surface — none have enough energy to cause emission.
Physicists measure the stopping potential required to halt emitted photoelectrons for various light frequencies and intensities.
At constant frequency, increasing intensity raises photocurrent but leaves the stopping potential unchanged.
At higher frequencies, the stopping potential increases proportionally to frequency.
This confirms that photon energy (and therefore maximum kinetic energy) depends solely on light frequency, not intensity.
Not all photoelectrons are emitted from identical energy states within the metal.
Some originate deeper below the surface and lose energy escaping the metal, while others start near the surface and retain the most energy.
Thus, photoelectrons exhibit a range of kinetic energies, with the fastest — those escaping most efficiently — having the maximum kinetic energy used in Einstein’s photoelectric equation.
Changing the metal alters the work function, which affects the threshold frequency but not the independence of kinetic energy from intensity.
Metals with lower work functions emit electrons at lower frequencies.
For any given frequency above threshold, increasing intensity still only increases the emission rate, not the maximum kinetic energy.
This behaviour is universal across all photoemissive metals, confirming the quantum model’s consistency.
Practice Questions
Question 1 (2 marks)
Explain why increasing the intensity of light incident on a metal surface does not increase the maximum kinetic energy of the emitted photoelectrons, provided the frequency of the light remains constant and above the threshold frequency.
Mark Scheme:
1 mark: States that each photoelectron is emitted as a result of a single photon–electron interaction.
1 mark: States that the energy of each photon depends only on its frequency, so increasing intensity increases the number of photons (and therefore emitted electrons) but not their individual energies.
Question 2 (5 marks)
An experiment is carried out to investigate the photoelectric effect. Monochromatic light of constant frequency, greater than the threshold frequency of the metal, is incident on a clean metal surface. The intensity of the light is gradually increased.
(a) Describe what happens to:
(i) the rate of emission of photoelectrons (2 marks)
(ii) the maximum kinetic energy of the emitted photoelectrons (1 mark)
(b) Explain your observations using the photon model of light. (2 marks)
Mark Scheme:
(a)(i)
1 mark: States that the rate of emission of photoelectrons increases with intensity.
1 mark: Explains this is because more photons strike the surface per second, so more electrons are emitted per second.
(a)(ii)
1 mark: States that the maximum kinetic energy remains unchanged because it depends only on photon frequency.
(b)
1 mark: States that each photoelectron absorbs energy from a single photon.
1 mark: States that the energy of a photon (E = hf) depends only on frequency, not intensity; therefore, photon energy and hence maximum kinetic energy remain constant.

