OCR Specification focus:
‘Above threshold, emission rate is directly proportional to incident radiation intensity.’
When electromagnetic radiation of sufficient frequency strikes a metal surface, electrons may be emitted in a phenomenon known as the photoelectric effect. Once the frequency of the incident light exceeds the threshold frequency, increasing its intensity affects only the rate of emission, not the energy of the emitted electrons. This subsubtopic explores the relationship between intensity and emission rate in photoelectric phenomena.
The Role of Intensity in the Photoelectric Effect
The intensity of electromagnetic radiation refers to the power per unit area incident on a surface. In the context of the photoelectric effect, this determines how many photons strike the metal per second per unit area.
Increasing the intensity of light increases the number of photons incident per second, but does not affect the energy of each photon, which depends only on its frequency.
Photon: A discrete packet of electromagnetic energy, each carrying energy proportional to its frequency according to E = hf, where h is Planck’s constant.
Therefore, if the frequency is above the threshold frequency (meaning each photon has enough energy to overcome the work function of the metal), an increase in intensity leads to a greater emission rate — more electrons emitted per second.
The Threshold Frequency and Its Importance
The threshold frequency is the minimum frequency of incident radiation required to eject electrons from the surface of a given material. Below this frequency, no electrons are emitted, regardless of the intensity of the light.
Threshold Frequency (f₀): The minimum frequency of incident electromagnetic radiation required to liberate electrons from a metal surface.
For frequencies below f₀, increasing intensity merely increases the number of photons that are individually too low in energy to cause emission. The process remains impossible because photon energy cannot be shared or accumulated by an electron from multiple photons — the interaction is strictly one photon to one electron.
Above the Threshold Frequency: Intensity Determines Emission Rate
Once the frequency of the incident light exceeds f₀, every photon has sufficient energy to cause electron emission, provided that it collides with a surface electron.
More intense light (higher photon flux) means more photons per second reach the metal surface.
Since each photon can eject one electron, the rate of emission increases proportionally with intensity.
However, the maximum kinetic energy of emitted electrons remains unchanged for a fixed frequency because photon energy (hf) is constant.
Thus, intensity affects emission rate, not the energy of the emitted electrons.
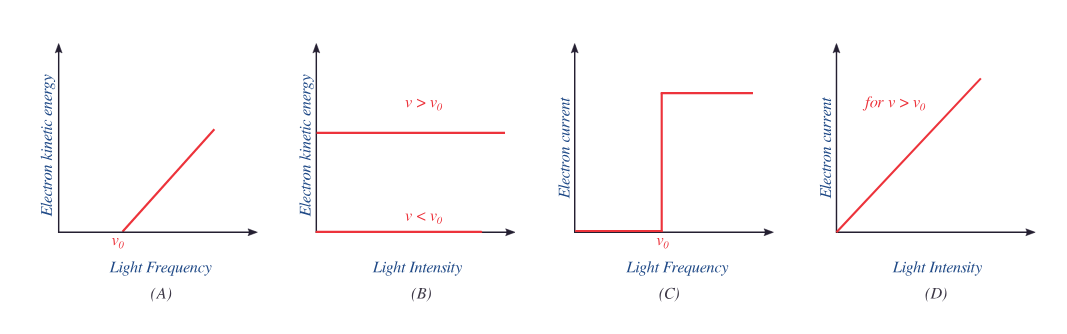
Schematic summary of photoelectric trends. Panel (D) shows photocurrent directly proportional to light intensity above threshold, confirming that emission rate increases linearly while kinetic energy remains unaffected. Panels (A–C) include extra frequency-related detail beyond this subsubtopic but reinforce conceptual understanding. Source.
EQUATION
—-----------------------------------------------------------------
Einstein’s Photoelectric Equation: hf = Φ + KEₘₐₓ
h = Planck’s constant (6.63 × 10⁻³⁴ J·s)
f = frequency of incident light (Hz)
Φ = work function of the metal (J)
KEₘₐₓ = maximum kinetic energy of emitted electrons (J)
—-----------------------------------------------------------------
This equation reinforces that increasing intensity does not alter f, and therefore KEₘₐₓ remains constant for a given frequency.
Photon Flux and Emission Rate
The term photon flux quantifies the number of photons striking the surface per second per unit area.
Photon Flux: The number of incident photons per unit area per unit time, proportional to the light’s intensity and inversely proportional to photon energy.
If the frequency remains constant and above f₀, increasing intensity increases the photon flux, leading to a proportional rise in the rate of emitted electrons. The relationship can be summarised as:
High intensity, above threshold frequency → High emission rate.
Low intensity, above threshold frequency → Low emission rate.
Any intensity, below threshold frequency → No emission at all.
This linear relationship between intensity and emission rate continues until other limiting factors (such as depletion of surface electrons or space charge effects) become significant, which are beyond the scope of the A-Level syllabus.
Distinguishing Between Intensity and Energy Effects
Students must clearly separate photon energy from light intensity:
Photon energy (hf) depends solely on frequency and determines whether emission can occur.
Intensity controls how many photons (and therefore electrons) interact per second.
An important conceptual distinction is that classical wave theory predicted increased intensity should increase electron energy, but experiments showed only the number of emitted electrons increased. This provided strong evidence for the photon model of light, which treats light as discrete particles rather than continuous waves.
Experimental Observations Supporting the Relationship
In photoelectric experiments using metals like zinc or sodium:
When monochromatic light (single frequency) above the threshold frequency shines on the metal, the rate of electron emission is observed to increase linearly with intensity.
Photoelectric apparatus schematic showing a light source illuminating a metal cathode in a vacuum tube. Emitted electrons travel to the anode, and the resulting current measures the emission rate. Adjusting light intensity changes the photocurrent while frequency remains fixed above threshold. Source.
The maximum kinetic energy of electrons, measured using stopping potential methods, remains unchanged when intensity changes but increases with frequency.
When light below the threshold frequency is used, no emission occurs, even at high intensities.
These results confirm that each photoelectron originates from a one-to-one photon–electron interaction, and that emission rate depends directly on how many such interactions occur per second, i.e., on intensity.
Applications and Implications
Understanding the dependence of emission rate on intensity is fundamental in technologies that exploit photoelectric principles:
Photodiodes and photoelectric sensors rely on the proportional relationship between light intensity and emission rate to measure illumination accurately.
Solar cells, while more complex due to semiconductor effects, also depend on photon absorption rates linked to intensity.
Photomultiplier tubes amplify the small photoelectric current generated by weak light, where intensity control determines the initial electron count.
These applications demonstrate how control of intensity above threshold frequency enables precise manipulation of photoelectric current.
Summary of Key Relationships
Emission occurs only if frequency ≥ threshold frequency (f₀).
Above threshold, increasing intensity increases the number of emitted electrons per second (emission rate).
Maximum kinetic energy of photoelectrons depends on frequency, not intensity.
The observed dependence supports the quantum (photon) model of light.
FAQ
Below the threshold frequency, each photon’s energy is insufficient to overcome the metal’s work function.
Increasing intensity only increases the number of low-energy photons striking the surface per second, not their individual energy.
Since electrons cannot combine energy from multiple photons, no emission occurs regardless of intensity.
The emission rate is determined by measuring the photocurrent using a sensitive ammeter in a circuit connected to the photoemissive surface.
The photocurrent is directly proportional to the number of electrons emitted per second.
By varying the light intensity, experimenters observe a corresponding change in photocurrent, confirming the proportional relationship.
The measurement is taken while maintaining a constant frequency above the threshold to isolate the intensity effect.
At very high intensities, several limiting factors can appear:
Surface electron availability: The number of free electrons near the surface that can escape becomes limited.
Space charge effects: Emitted electrons can form a cloud that repels further emission.
Saturation current: Once all available electrons are emitted as soon as photons arrive, the current reaches a plateau.
Thus, while emission rate is proportional to intensity initially, it can level off at extremely high intensities.
Classical theory predicted that increasing intensity should increase electron energy, as energy was thought to accumulate gradually from the wave.
In contrast, experiments showed that increasing intensity only increased the number of emitted electrons, while their maximum kinetic energy remained constant.
This contradiction proved that energy transfer occurs in discrete quanta (photons), each with energy dependent solely on frequency.
Yes, the surface material influences the work function and hence the threshold frequency.
Once the threshold condition is satisfied, the proportional relationship between intensity and emission rate still holds, but:
Metals with lower work functions emit more easily, showing higher emission rates at the same intensity.
Surface cleanliness and oxidation can reduce emission efficiency by absorbing or scattering photons.
The general trend—rate proportional to intensity above threshold—remains valid across materials.
Practice Questions
Question 1 (2 marks)
A beam of monochromatic light with frequency greater than the threshold frequency is shone on a clean metal surface.
Explain what happens to the rate of emission of photoelectrons when the intensity of the light is increased, and why the maximum kinetic energy of the emitted electrons does not change.
Mark scheme:
1 mark: States that the rate of emission (number of emitted electrons per second) increases when intensity is increased.
1 mark: States that maximum kinetic energy remains constant because it depends only on photon energy (frequency), not on intensity.
Question 2 (5 marks)
In a photoelectric experiment, monochromatic light of constant frequency above the threshold frequency is directed onto a metal surface.
Describe and explain the effect of increasing the light intensity on:
(a) the number of electrons emitted per second,
(b) the maximum kinetic energy of the emitted electrons,
and discuss how these observations provide evidence for the photon model of electromagnetic radiation.
Mark scheme:
1 mark: States that increasing intensity increases the number of photons per second incident on the metal.
1 mark: States that each photon can release one electron, so the number of emitted electrons per second increases (emission rate rises).
1 mark: States that the maximum kinetic energy of emitted electrons remains unchanged because photon energy depends only on frequency (E = hf).
1 mark: Explains that this result contradicts the classical wave theory, which predicted increased energy with higher intensity.
1 mark: Concludes that the results support the photon (quantum) model, where light consists of discrete energy packets transferring energy in one-to-one interactions with electrons.

