OCR Specification focus:
‘Matter waves satisfy λ = h/p; link momentum and wavelength for particles.’
The de Broglie wavelength reveals that all matter exhibits both wave-like and particle-like behaviour, connecting momentum to wavelength and extending quantum theory to material particles.
The de Broglie Hypothesis
In 1924, Louis de Broglie proposed a revolutionary idea: if light, once thought to be purely a wave, could display particle properties, then perhaps particles such as electrons could also display wave-like characteristics. This insight unified the concepts of waves and particles under one framework, laying the groundwork for wave–particle duality in quantum physics.
The Concept of Matter Waves
De Broglie suggested that every particle with momentum has an associated wave whose wavelength depends on that momentum. This means that even solid, tangible particles can be described by wave properties under the right conditions.
Matter Waves: The theoretical waves associated with moving particles, where the wavelength is inversely proportional to the particle’s momentum.
These waves are not visible light or mechanical vibrations but probability waves, describing the likelihood of finding a particle in a given position.
The de Broglie Wavelength Equation
De Broglie’s relationship between a particle’s momentum and its associated wavelength forms a cornerstone of modern quantum mechanics.
EQUATION
—-----------------------------------------------------------------
de Broglie Wavelength (λ) = h / p
λ = Wavelength of the particle (metres, m)
h = Planck’s constant (6.63 × 10⁻³⁴ joule seconds, J s)
p = Momentum of the particle (kilogram metres per second, kg m/s)
—-----------------------------------------------------------------
The equation shows that as a particle’s momentum increases, its wavelength decreases. Hence, heavier or faster-moving particles have extremely small wavelengths, making their wave-like nature unobservable in everyday life.
Linking Momentum and Wavelength
Momentum, defined as the product of mass and velocity, directly determines the spatial extent of a particle’s wave properties. For particles with low mass and high velocity, the de Broglie wavelength becomes comparable to the atomic scale, allowing their wave nature to manifest through diffraction and interference effects.
EQUATION
—-----------------------------------------------------------------
Momentum (p) = m × v
m = Mass of the particle (kilograms, kg)
v = Velocity of the particle (metres per second, m/s)
—-----------------------------------------------------------------
Substituting this expression for momentum into the de Broglie equation gives λ = h / (m v). This relation allows scientists to predict when a particle’s wave behaviour will become experimentally observable.
Experimental Evidence for Matter Waves
The concept of matter waves was confirmed through electron diffraction experiments, where electrons, when passed through a thin crystal or polycrystalline graphite, produced diffraction patterns characteristic of waves.
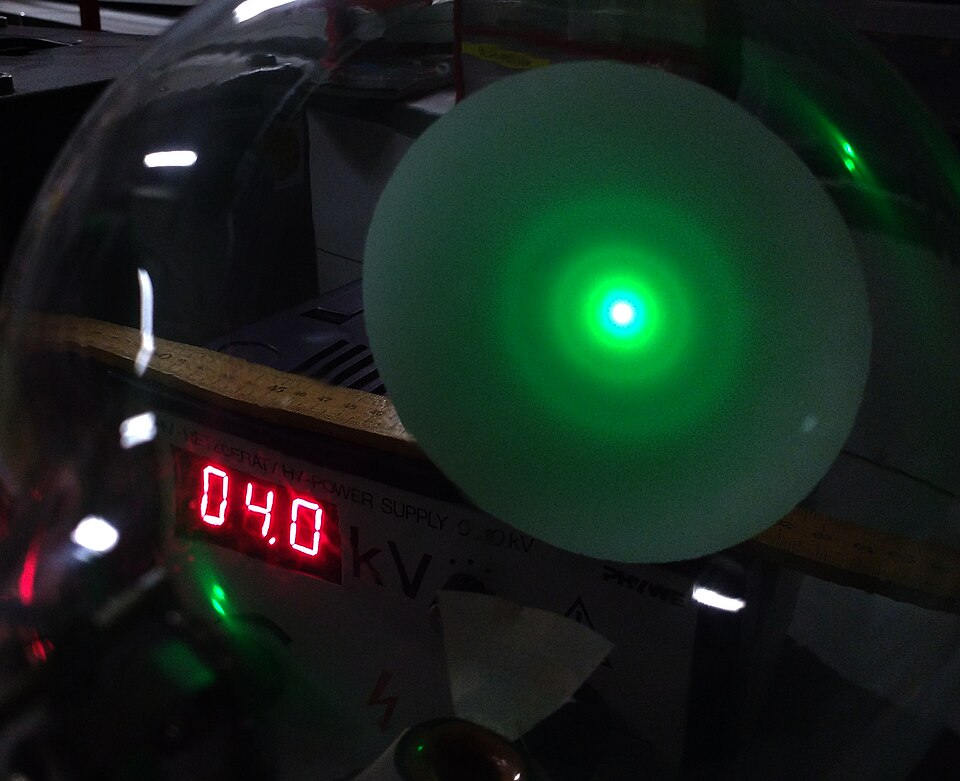
Electron diffraction pattern from polycrystalline graphite showing concentric rings on a fluorescent screen. The rings arise from Bragg diffraction of electrons whose de Broglie wavelength matches spacings in the graphite lattice. This is direct, visual evidence of wave–particle duality. Source.
These experiments validated de Broglie’s hypothesis by demonstrating that particles could interfere and diffract, behaviours previously attributed only to waves.
Such observations strengthened the idea that wave–particle duality applies universally, not just to light.
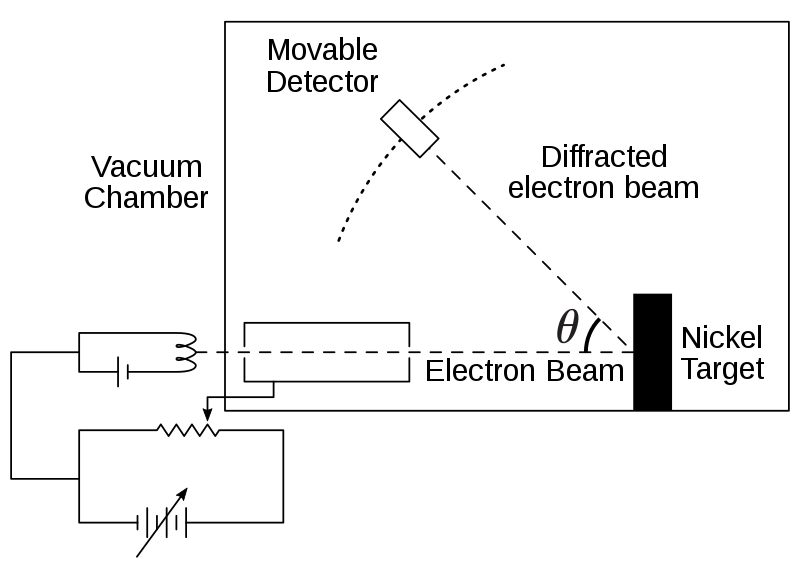
Davisson–Germer electron diffraction apparatus: an electron gun directs a beam onto a crystalline target, and a detector measures intensity versus angle, revealing diffraction maxima. This landmark experiment confirmed de Broglie’s hypothesis by showing electrons behave as waves. (Includes apparatus detail not required by OCR but aids visualisation.) Source.
Key outcomes from these experiments include:
Confirmation that λ = h/p accurately predicts observed diffraction patterns.
The demonstration that wave-like behaviour becomes significant when λ is comparable to atomic or molecular spacings.
Reinforcement that quantum mechanics must describe both the motion and spatial probability of particles.
Relationship to Quantum Theory
De Broglie’s proposal inspired the development of the Schrödinger equation, which mathematically describes matter waves and underpins quantum mechanics.
In this view, the wavefunction (ψ) represents the probability amplitude of locating a particle, and the square of its magnitude gives the probability density.
Thus, de Broglie’s wavelength links a physical property — momentum — to a statistical description of particle location.
This dual description resolved earlier inconsistencies between classical mechanics and quantum observations, uniting energy quantisation, the photoelectric effect, and electron behaviour within atoms.
Practical Implications and Limits
While all objects technically possess a de Broglie wavelength, only those with very small mass (like electrons, neutrons, or atoms) and moderate velocities exhibit measurable wave properties.
Observable vs. Unobservable Diffraction
Wave behaviour can be observed only when the de Broglie wavelength is comparable to or larger than the interatomic spacing in a diffracting material, typically around 10⁻¹⁰ m.
For macroscopic objects, such as a tennis ball, the wavelength is minuscule (on the order of 10⁻³⁴ m), rendering any wave phenomena undetectable.
Bullet points summarising observability:
Microscopic particles (e.g. electrons, neutrons): Wavelengths in the range of atomic spacing → observable diffraction.
Macroscopic bodies (e.g. everyday objects): Wavelengths far too small → wave effects negligible.
Intermediate cases (e.g. large molecules): Specialised experiments required to detect interference patterns.
Key Features of the de Broglie Relationship
Establishes a universal connection between particle motion and wave properties.
Introduces the concept that all matter exhibits wave–particle duality.
Forms a quantitative link between classical momentum and quantum wavelength.
Provides the basis for electron diffraction and quantum mechanical models of atoms.
Demonstrates that wave behaviour diminishes with increasing mass or speed.
Enables estimation of wave-like behaviour in different systems through measurable quantities.
Summary of Quantum Significance
The de Broglie wavelength is fundamental to understanding the microscopic world, illustrating how particles exhibit both wave and particle aspects.
It connects directly to the quantised nature of energy and motion, showing that even material particles obey the same fundamental constants governing photons and electromagnetic waves.
This concept marks a turning point in physics — bridging classical mechanics and quantum theory, and defining the framework for understanding atomic and subatomic behaviour.
FAQ
The main factors are the accelerating voltage, target material quality, and vacuum conditions.
A precisely known voltage ensures accurate calculation of electron momentum.
Using a uniform, thin polycrystalline film (like graphite) gives clear, consistent ring patterns.
Good vacuum levels prevent electrons scattering off air molecules, maintaining sharp diffraction rings.
Improper alignment of the electron beam or magnetic interference can also distort measurements and reduce precision.
The de Broglie hypothesis explains why only certain electron orbits are stable in atoms.
When an electron’s orbital circumference equals an integer multiple of its wavelength, a standing wave forms, preventing destructive interference.
This idea supports quantised energy levels, since only specific wavelengths — and thus energies — fit stable orbits. It was crucial in refining the Bohr model into modern quantum theory.
Yes, but only under carefully controlled conditions.
Neutrons, atoms, and even molecules can exhibit diffraction if their velocities are low enough to give wavelengths comparable to atomic spacings.
Experiments using C₆₀ fullerenes (large molecules) have demonstrated measurable interference patterns.
However, as particle mass increases, λ becomes extremely small, demanding advanced equipment like matter-wave interferometers or ultra-high vacuum systems to detect it.
Temperature influences particle kinetic energy and hence momentum.
Higher temperature → higher average velocity → larger momentum → shorter wavelength.
Lower temperature → lower velocity → longer wavelength.
In ultracold experiments, cooling atoms to near absolute zero can increase their wavelengths enough to overlap, producing phenomena like Bose–Einstein condensation, where particles behave as a single wave.
The Planck constant (h) bridges the quantum and macroscopic worlds by linking wave properties to particle momentum.
It defines the scale at which quantum effects become significant — the smaller h is relative to the system, the less noticeable wave-like behaviour becomes.
Thus, h determines why electrons display observable diffraction while everyday objects do not, as their enormous momenta make h/p vanishingly small.
Practice Questions
Question 1 (2 marks)
State the de Broglie relationship for a particle and explain how the wavelength of a particle changes as its momentum increases.
Mark Scheme:
1 mark: Correctly states the relationship λ = h / p (accept λ = h / mv).
1 mark: Explains that as momentum increases, the wavelength decreases (inverse relationship).
Question 2 (5 marks)
Electrons accelerated through a potential difference of several thousand volts are directed at a thin graphite film and produce a diffraction pattern.
(a) Explain why this observation provides evidence for the wave–particle duality of electrons. (3 marks)
(b) Using the de Broglie equation, discuss why similar diffraction effects are not observed for macroscopic objects such as tennis balls. (2 marks)
Mark Scheme:
(a)
1 mark: States that diffraction is a property of waves.
1 mark: Notes that the appearance of a diffraction pattern shows electrons behaving as waves.
1 mark: Recognises that electrons are particles, hence showing both particle and wave properties (wave–particle duality).
(b)
1 mark: Refers to the de Broglie equation λ = h / p (or λ = h / mv).
1 mark: Explains that macroscopic objects have very large mass and therefore very small λ (too small to observe diffraction effects).

